The global antipsychotic drugs market, valued at USD 18.1 billion in 2023, is projected to expand at a compound annual growth rate (CAGR) of 2.9% through 2030, driven by rising prevalence of psychiatric disorders and increased healthcare expenditure, according to Grand View Research. Within this landscape, zuclopenthixol—an atypical antipsychotic used primarily in the management of schizophrenia and acute psychotic episodes—remains a key therapeutic option, particularly in European and select Asian markets. As demand for effective, long-acting psychiatric treatments grows, a select group of manufacturers has emerged as leading producers of zuclopenthixol, leveraging established formulations such as zuclopenthixol dihydrochloride and decanoate. These companies benefit from strong regulatory footholds, robust production capabilities, and ongoing partnerships in mental health supply chains. Based on market availability, manufacturing scale, and regulatory compliance, here are the top six zuclopenthixol manufacturers shaping the current pharmaceutical landscape.
Top 6 Zuclopenthixol Manufacturers 2026
(Ranked by Factory Capability & Trust Score)
#1 Zuclopenthixol suppliers & manufacturers in China
Domain Est. 2006
Website: chemicalbook.com
Key Highlights: Zuclopenthixol is a kind of antipsychotic agent. It is a thioxanthene-based neuroleptic with in vivo action similar to the phenothiazine antipsychotics….
#2 Zuclopenthixol Decanoate API Manufacturers
Domain Est. 2014
Website: pharmacompass.com
Key Highlights: Click here to find a list of Zuclopenthixol Decanoate manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass….
#3 Products
Domain Est. 1996
Website: lundbeck.com
Key Highlights: We have a wide range of products that help treat and prevent brain diseases. Lundbeck has products registered in over 100 countries around the world….
#4 Zuclopenthixol
Domain Est. 1997
Website: pubchem.ncbi.nlm.nih.gov
Key Highlights: Zuclopenthixol is a thioxanthene-based neuroleptic with therapeutic actions similar to the phenothiazine antipsychotics. It is an antagonist at D1 and D2 ……
#5 Details for: CLOPIXOL
Domain Est. 2014
Website: hpr-rps.hres.ca
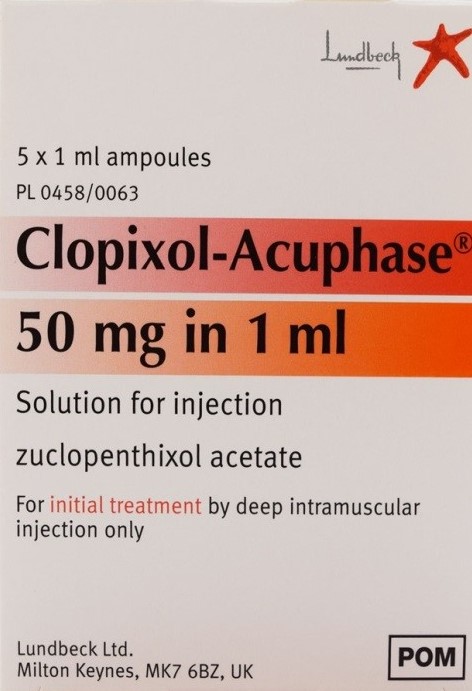
Key Highlights: Each Clopixol® contains Zuclopenthixol Tablets Lundbeck Std. (as hydrochloride). Clopixol-Acuphase injection contains zuclopenthixol acetate. Clopixol Depot ……
#6 ZUCLOPENTHIXOL ACETATE
Website: sfda.gov.sa
Key Highlights: Scientific Name ; Trade Name. CLOPIXOL-ACUPHASE 50MG-ML OILY AMP ; Other Info. Agent Name. SAUDI INTERNATIONAL TRADING COMPANY LTD (SITCO)….
Expert Sourcing Insights for Zuclopenthixol
H2: 2026 Market Trends for Zuclopenthixol
As of 2026, the market for zuclopenthixol, a first-generation (typical) antipsychotic primarily used in the management of schizophrenia and acute psychotic episodes, reflects broader shifts in psychiatric treatment, healthcare policy, and pharmaceutical innovation. While zuclopenthixol remains a niche agent due to its side effect profile and the dominance of atypical antipsychotics, several key trends shape its role and market dynamics:
-
Declining Use in High-Income Countries
In North America, Western Europe, and parts of Asia-Pacific, the use of zuclopenthixol continues to decline. This is largely due to the widespread preference for second-generation antipsychotics (SGAs) such as risperidone, olanzapine, and long-acting injectables like aripiprazole. SGAs are generally associated with lower risks of extrapyramidal symptoms (EPS) and tardive dyskinesia—side effects more commonly linked to zuclopenthixol. As a result, zuclopenthixol is increasingly reserved for treatment-resistant cases or specific clinical scenarios (e.g., rapid tranquilization with zuclopenthixol acetate). -
Stable or Niche Use in Low- and Middle-Income Countries (LMICs)
In contrast, zuclopenthixol—particularly its depot form (zuclopenthixol decanoate)—retains relevance in regions with limited healthcare budgets, such as parts of Eastern Europe, Latin America, and Africa. Its low cost, long duration of action, and established efficacy make it a pragmatic choice where access to newer, more expensive antipsychotics is restricted. In these markets, it continues to serve as a key option for long-term maintenance therapy in schizophrenia. -
Shift Toward Personalized and Safer Psychopharmacology
The 2026 mental health landscape emphasizes precision psychiatry and reduced treatment burden. Pharmacogenomic testing and digital therapeutics are increasingly used to guide antipsychotic selection. Zuclopenthixol, lacking strong pharmacogenetic data and carrying a less favorable metabolic and neurological safety profile, is less likely to be selected in personalized treatment algorithms. This trend further marginalizes its use in modern psychiatric practice. -
Generic Dominance and Low Market Value
Zuclopenthixol is available only as a generic medication, with no branded formulations remaining under patent. This has led to a highly competitive, low-margin market. As a result, major pharmaceutical companies have shown minimal interest in developing new formulations or investing in clinical trials for zuclopenthixol. Its market value remains small, with limited growth potential. -
Regulatory and Safety Scrutiny
Regulatory agencies, including the EMA and national health authorities, continue to emphasize risk minimization for typical antipsychotics. Warnings regarding QT prolongation, neuroleptic malignant syndrome (NMS), and EPS are well-documented for zuclopenthixol. In 2026, these safety concerns contribute to restrictive prescribing guidelines, further limiting its adoption—especially in elderly patients or those with comorbid medical conditions. -
Role in Crisis and Forensic Psychiatry
One area where zuclopenthixol maintains clinical utility is in acute psychiatric settings. Zuclopenthixol acetate (Clopixol Acuphase) is still used in some countries for rapid sedation of agitated patients, particularly in forensic or emergency psychiatry. However, even here, alternatives like intramuscular olanzapine or lorazepam combinations are increasingly favored due to faster onset and better safety.
Conclusion
By 2026, zuclopenthixol occupies a shrinking but persistent niche in global antipsychotic therapy. While its use is declining in advanced healthcare systems due to safety concerns and the availability of superior alternatives, it remains a cost-effective option in resource-limited settings. Market trends suggest continued marginalization in favor of newer, better-tolerated agents, with no significant commercial or therapeutic resurgence expected. Its legacy persists in specific clinical contexts, but it is no longer a frontline treatment in modern psychiatry.

Common Pitfalls in Sourcing Zuclopenthixol: Quality and Intellectual Property Concerns
Sourcing zuclopenthixol, a thioxanthene antipsychotic used primarily in the management of schizophrenia and acute psychosis, involves navigating significant challenges related to product quality and intellectual property (IP) rights. Failure to address these pitfalls can lead to regulatory non-compliance, patient safety risks, and legal exposure.
Quality-Related Pitfalls
Ensuring the consistent quality of zuclopenthixol—whether sourced as an active pharmaceutical ingredient (API) or finished dosage form—is paramount. Common quality pitfalls include:
-
Inadequate Supplier Qualification: Engaging manufacturers without thorough audits of their Good Manufacturing Practice (GMP) compliance increases the risk of receiving substandard or contaminated product. Suppliers from regions with weak regulatory oversight may lack robust quality control systems.
-
Impurity Profile Variability: Zuclopenthixol can degrade or form impurities during synthesis and storage. Poor manufacturing processes may result in unacceptable levels of genotoxic or otherwise harmful impurities, especially if specifications are not aligned with ICH guidelines.
-
Counterfeit or Adulterated Material: The psychiatric drug market is vulnerable to counterfeit products. Sourcing through unverified channels may result in receiving falsified zuclopenthixol with incorrect potency, wrong excipients, or even inert or harmful substances.
-
Instability and Improper Storage: Zuclopenthixol, particularly in long-acting injectable formulations (e.g., zuclopenthixol decanoate), is sensitive to temperature and light. Poor handling during transportation or storage can compromise stability and efficacy.
-
Lack of Batch Traceability: Inadequate documentation or fragmented supply chains may hinder full traceability, complicating recalls and pharmacovigilance efforts in case of quality issues.
Intellectual Property-Related Pitfalls
Zuclopenthixol is a legacy compound, but IP considerations remain critical, especially when sourcing generic versions or developing new formulations:
-
Patent Expiry Misinterpretation: While the core compound patents for zuclopenthixol have expired, secondary patents covering specific formulations, delivery systems (e.g., depot injections), or manufacturing processes may still be in force. Sourcing or marketing a product without verifying freedom-to-operate risks patent infringement.
-
Data Exclusivity and Regulatory Protection: In some jurisdictions, regulatory data protection may extend market exclusivity beyond patent life. Launching a generic version too early—even after patent expiry—could violate these protections, leading to legal challenges.
-
Geographical IP Variability: IP status varies by country. A product considered generic in one market may still be under patent or regulatory protection in another. Sourcing globally without jurisdiction-specific IP due diligence can result in customs seizures or litigation.
-
Infringement via Manufacturing Process: Even if the final product is off-patent, using a patented synthesis method to manufacture zuclopenthixol API can constitute infringement. Sourcing from manufacturers who rely on protected processes exposes the buyer to liability.
-
Trademark Confusion: Brand names for zuclopenthixol (e.g., Cisordinol, Clopixol) are trademark-protected. Using similar names or packaging for sourced generics may lead to trademark disputes, especially in regions with strong brand enforcement.
In summary, successful sourcing of zuclopenthixol requires rigorous quality assurance protocols and comprehensive IP landscape analysis to mitigate risks related to product integrity, regulatory compliance, and legal liability.
Logistics & Compliance Guide for Zuclopenthixol
Overview of Zuclopenthixol
Zuclopenthixol is a thioxanthene-class antipsychotic medication used primarily in the treatment of schizophrenia and other acute psychotic disorders. It is available in various formulations, including oral tablets and long-acting intramuscular injections (e.g., zuclopenthixol acetate and zuclopenthixol decanoate). Due to its potency and potential for serious side effects, strict logistical and compliance protocols must be followed during handling, storage, distribution, and administration.
Regulatory Classification
Zuclopenthixol is classified as a prescription-only medication (POM) in most jurisdictions. In certain countries, it may be subject to additional controls under mental health or psychotropic substance regulations. For example:
– In the UK, it is a Prescription-Only Medicine regulated under the Human Medicines Regulations 2012.
– In the US, although not FDA-approved, it may be available via special access programs and is treated as a controlled substance in certain contexts due to its use in psychiatric care.
– Internationally, it falls under the World Health Organization’s (WHO) guidelines for psychotropic substances, requiring secure handling and documentation.
Storage Requirements
Proper storage is critical to maintain zuclopenthixol’s stability and efficacy:
– Temperature: Store between 15°C and 30°C (59°F–86°F) unless otherwise specified by the manufacturer. Avoid freezing.
– Light Protection: Keep in original packaging to protect from light, especially for injectable forms.
– Security: Store in a locked, access-controlled area to prevent unauthorized access, particularly in clinical or pharmacy settings.
– Separation: Keep separate from incompatible substances and food products.
Transportation and Distribution
Transportation of zuclopenthixol must comply with pharmaceutical logistics standards:
– Use validated cold chain methods if required by formulation (especially for liquid injectables).
– Ensure tamper-evident packaging is intact upon receipt and dispatch.
– Maintain a documented chain of custody, particularly for high-dose or depot formulations.
– Distribute only to authorized healthcare providers or facilities with proper prescribing credentials.
Handling and Dispensing
- Authorized Personnel: Only licensed healthcare professionals or registered pharmacists should dispense or administer zuclopenthixol.
- Prescription Verification: Confirm validity of prescription, including patient details, dosage, and prescriber authorization.
- Labeling: Ensure all dispensed products are correctly labeled with patient name, drug name, strength, dosage instructions, and expiry date.
- Patient Counseling: Provide appropriate information on side effects, adherence, and monitoring requirements, particularly for depot injections.
Administration and Clinical Use
- Parenteral Use: Zuclopenthixol acetate (fast-acting) and decanoate (long-acting) must be administered by trained medical staff via deep intramuscular injection.
- Monitoring: Patients require regular clinical and laboratory monitoring for adverse effects (e.g., extrapyramidal symptoms, sedation, QT prolongation).
- Emergency Protocols: Facilities must have protocols in place for managing overdose or acute adverse reactions (e.g., neuroleptic malignant syndrome).
Recordkeeping and Documentation
- Maintain detailed records of all transactions involving zuclopenthixol, including:
- Prescription logs
- Dispensing records
- Inventory audits
- Adverse event reports
- Retain records for a minimum of 2–5 years, depending on national regulations.
- Report any loss, theft, or suspected diversion immediately to relevant authorities (e.g., pharmacy board, health department).
Disposal and Waste Management
- Follow local hazardous pharmaceutical waste regulations for expired or unused zuclopenthixol.
- Do not dispose of in household trash or sewage systems.
- Use authorized pharmaceutical waste disposal services; syringes and vials must be placed in designated sharps containers.
Compliance and Auditing
- Conduct regular internal audits to ensure adherence to storage, handling, and documentation protocols.
- Train all relevant staff annually on compliance requirements, including controlled substance handling and patient safety.
- Prepare for inspections by regulatory bodies (e.g., MHRA in the UK, EMA in Europe) with up-to-date policies and records.
Special Considerations
- Depot Injections: Extra vigilance is required due to long half-life and risk of cumulative toxicity. Confirm patient identity and injection site meticulously.
- High-Risk Patients: Monitor elderly or medically compromised patients closely; dose adjustments may be necessary.
- International Shipments: If exporting/importing, comply with international drug control treaties and obtain necessary import/export licenses.
Conclusion
Zuclopenthixol is an effective antipsychotic requiring stringent logistical and compliance measures due to its potency and regulatory status. Adherence to storage, handling, documentation, and administration guidelines ensures patient safety, legal compliance, and uninterrupted access to treatment. Regular training and audits are essential to maintain high standards across the supply and care chain.
Zuclopenthixol is a potent antipsychotic medication used primarily in the management of schizophrenia and other severe psychotic disorders. Due to its classification as a controlled substance in many countries, sourcing zuclopenthixol must be conducted strictly in accordance with local and international regulations. It should only be obtained through licensed pharmaceutical suppliers, authorized healthcare providers, or registered pharmacies with appropriate prescriptions. Illicit sourcing or unauthorized distribution poses serious legal, ethical, and health risks, including potential misuse, incorrect dosing, and adverse effects. Therefore, responsible sourcing of zuclopenthixol must prioritize patient safety, regulatory compliance, and professional medical oversight.