The global sun care market is experiencing robust growth, driven by rising consumer awareness of skin health and increasing demand for clean, non-toxic formulations. According to Grand View Research, the global sunscreen market size was valued at USD 10.5 billion in 2022 and is projected to expand at a compound annual growth rate (CAGR) of 6.3% from 2023 to 2030. A notable shift within this expansion is the growing preference for mineral-based sunscreens—particularly those utilizing non-nano zinc oxide—due to their favorable safety profiles and minimal environmental impact. Concurrently, there is a rising interest in skincare formulations enriched with nourishing, bioavailable ingredients such as beef tallow, celebrated for its skin-identical lipids and moisturizing properties. This convergence of clean beauty and holistic skincare has catalyzed demand for non-toxic zinc oxide sunscreens that incorporate grass-fed beef tallow. In response, a new wave of manufacturers is emerging, combining ethical sourcing, transparent labeling, and dermatologically sound formulations. The following profile highlights nine leading manufacturers at the forefront of this niche, where efficacy meets ingredient integrity.
Top 9 Zinc Oxide Sunscreen Non Toxic Beef Tallow Manufacturers 2026
(Ranked by Factory Capability & Trust Score)
#1 Based Balm’s Tallow & Zinc Sun Balm
Domain Est. 2022
Website: basedbalm.com
Key Highlights: Out of stockThe Natural Tallow & Zinc Sun Balm is 20% Non-nano Zinc Oxide by volume. The Non Nano Zinc creates a physical barrier between you and the sun.Missing: toxic manufactur…
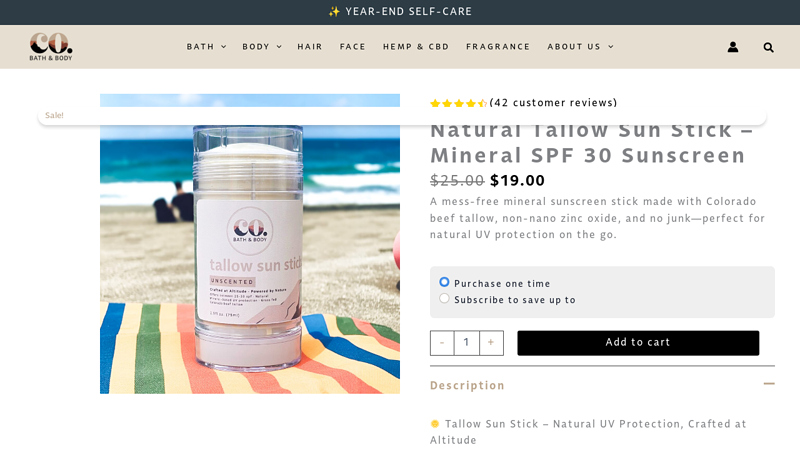
#2 Natural Tallow Sun Stick
Domain Est. 2022
Website: cobathandbody.com
Key Highlights: In stock Rating 4.6 (42) A mess-free mineral sunscreen stick made with Colorado beef tallow, non-nano zinc oxide, and no junk—perfect for natural UV protection on the go …Missi…
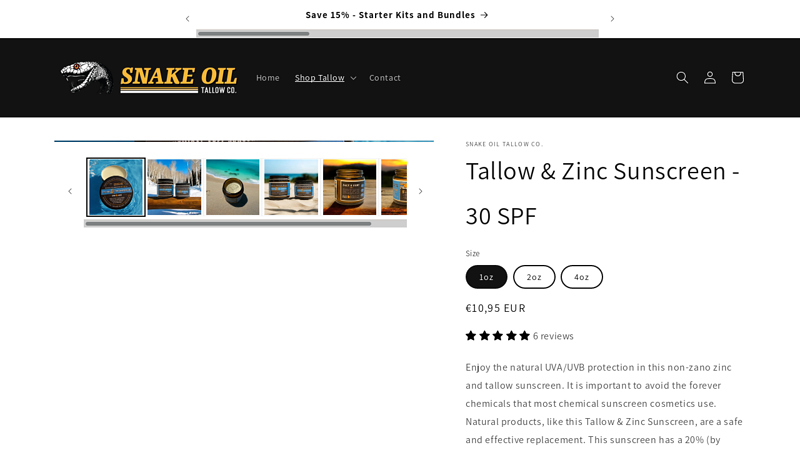
#3 Tallow & Zinc Sunscreen
Domain Est. 2023
Website: snakeoiltallow.com
Key Highlights: In stock Rating 5.0 (5) This sunscreen has a 20% (by weight) concentration of non-nano zinc oxide, making it equivalent to an SPF of 30. Use on face and body.Missing: toxic manu…
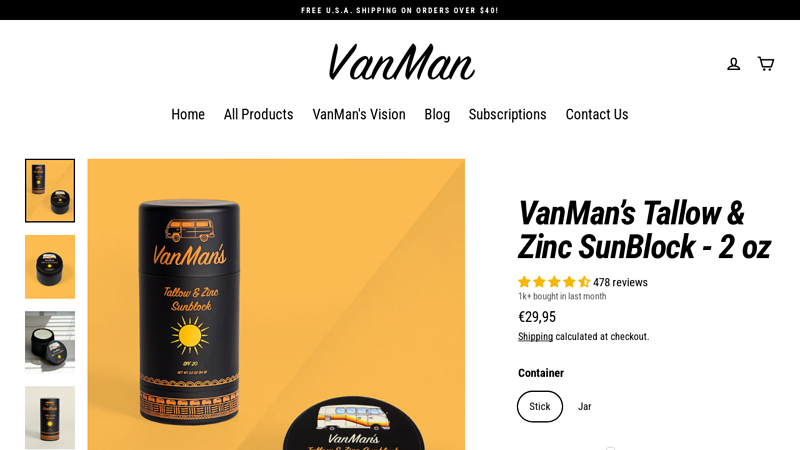
#4 VanMan’s Tallow & Zinc SunBlock
Domain Est. 2021
Website: vanman.shop
Key Highlights: VanMan’s sunblock uses non-nano Zinc Oxide to act as a mineral barrier. An SPF equivalent of about 20, water resistant, and nearly 100% absorbent….
#5 Tallow Sunscreens
Domain Est. 2023
#6 Eat My Face
Domain Est. 2023
Website: eatmyface.co
Key Highlights: Nourishing tallow sunscreen and beef tallow sunscreen with non-nano zinc oxide. Reef safe, mineral sunscreen SPF 30 for daily protection and recovery. Shop Now….
#7 Tallow Before Sun Cream
Domain Est. 2023
#8 Mineral Sun Balm – Now Available! Non
Domain Est. 2024
#9 Pure Mineral Sunscreen Designed for Active Families
Domain Est. 2024
Website: livetallowed.com
Key Highlights: In stock Rating 4.5 (2) Non-nano zinc oxide: Physical barrier protection from UVA/UVB rays; Grass-fed beef tallow: Moisturizes sun-exposed skin; Organic jojoba oil: Lightweight ….
Expert Sourcing Insights for Zinc Oxide Sunscreen Non Toxic Beef Tallow

H2: Market Trends for Zinc Oxide, Sunscreen, Non-Toxic, and Beef Tallow Products (2026 Outlook)
As consumer awareness around health, sustainability, and ingredient transparency intensifies, the personal care and skincare markets are undergoing significant transformation. By 2026, the convergence of demand for clean-label, non-toxic ingredients is driving growth in niche but rapidly expanding product categories—including zinc oxide sunscreens and beef tallow-based skincare. This analysis explores key market trends shaping the future of these products, with a focus on consumer behavior, regulatory shifts, innovation, and sustainability.
- Rising Demand for Non-Toxic Sunscreens with Zinc Oxide
Zinc oxide, a mineral UV filter, is poised to dominate the sunscreen market by 2026 due to increasing scrutiny over chemical UV filters like oxybenzone and octinoxate. Regulatory bodies in regions such as the EU and Hawaii have banned or restricted chemical sunscreens over environmental and health concerns, boosting demand for reef-safe, mineral-based alternatives. - Market Growth: The global mineral sunscreen market is projected to exceed $3.5 billion by 2026, with zinc oxide as the primary active ingredient.
- Consumer Preference: Clean beauty consumers favor non-nano zinc oxide for its broad-spectrum protection, low skin irritation potential, and biodegradability.
-
Innovation: Advances in formulation technology are reducing the white cast traditionally associated with zinc oxide, improving user acceptance.
-
Expansion of the “Non-Toxic” Skincare Movement
The definition of “non-toxic” continues to evolve, encompassing not only the absence of harmful chemicals but also ethical sourcing and environmental footprint. Brands emphasizing transparency, EWG verification, and cruelty-free certifications are gaining market share. - Retail Influence: Major retailers like Sephora, Credo Beauty, and Thrive Market are curating “clean” product lines, increasing accessibility.
-
Regulatory Trends: The U.S. FDA’s proposed sunscreen monograph revisions (2024–2026) may elevate zinc oxide and titanium dioxide as GRASE (Generally Recognized as Safe and Effective), further cementing their dominance.
-
Resurgence of Animal-Derived Actives: Beef Tallow in Skincare
Beef tallow, rendered from grass-fed, pasture-raised cattle, is emerging as a premium skincare ingredient due to its molecular similarity to human sebum and rich profile of fatty acids and fat-soluble vitamins (A, D, E, K). - Niche Market Growth: The tallow skincare market, though small, is growing at over 15% CAGR, driven by the “nose-to-tail” sustainability movement and interest in ancestral wellness practices.
- Target Demographics: Attracts paleo, biohacking, and regenerative agriculture communities seeking natural, nutrient-dense products.
-
Challenges: Scalability, odor control, and consumer perception remain barriers, but artisanal and indie brands are overcoming these through premium branding and traceability.
-
Convergence of Categories: Zinc Oxide + Beef Tallow Sunscreens
A novel product trend gaining traction is the fusion of zinc oxide with beef tallow in multi-functional sunscreens. These formulations offer UV protection while delivering deep moisturization and skin barrier support—appealing to consumers seeking minimal, effective routines. - Early Adopters: Brands like Fat and the Moon, Earth Sun Body, and Farmacy Beauty are piloting hybrid products.
-
Market Potential: While still emergent, the clean, regenerative skincare segment could see these hybrids capture a niche share by 2026, especially in direct-to-consumer (DTC) channels.
-
Sustainability and Regenerative Agriculture Drivers
Both zinc oxide and beef tallow benefit from alignment with broader environmental values: - Zinc Oxide: Preferred for its low aquatic toxicity and photostability.
- Beef Tallow: When sourced from regenerative farms, supports carbon sequestration and reduces waste by utilizing underused animal byproducts.
- Certifications: Demand for B-Corp, Regenerative Organic Certified (ROC), and Leaping Bunny labels will influence purchasing decisions.
Conclusion
By 2026, the market for zinc oxide sunscreens and non-toxic, beef tallow-based skincare products will be shaped by heightened consumer consciousness, regulatory clarity, and innovation in natural formulations. While zinc oxide dominates the mainstream mineral sunscreen space, beef tallow represents a high-potential niche ingredient, particularly when combined with clean sun protection. Brands that emphasize transparency, sustainability, and clinical efficacy will lead the convergence of these trends, capturing the loyalty of a growing base of health- and eco-conscious consumers.

Common Pitfalls When Sourcing Zinc Oxide Sunscreen Made with Non-Toxic Beef Tallow
Sourcing a high-quality, safe, and effective zinc oxide sunscreen formulated with non-toxic beef tallow presents unique challenges. While this combination offers potential benefits like natural sun protection and skin nourishment, several pitfalls must be navigated carefully to ensure product integrity, safety, and authenticity.
1. Zinc Oxide Quality and Particle Size Risks
One of the foremost concerns is ensuring the zinc oxide used is non-nano and non-coated, as these characteristics are critical for both safety and consumer trust in natural products.
- Nano-Particle Contamination: Many suppliers offer “non-nano” zinc oxide, but without rigorous third-party testing (e.g., electron microscopy or dynamic light scattering), it’s difficult to verify claims. Nano particles can potentially penetrate the skin and enter the bloodstream, contradicting the “non-toxic” promise.
- Coated vs. Uncoated Zinc Oxide: Coatings (e.g., dimethicone, alumina, stearic acid) improve dispersion and reduce whitening but may introduce synthetic or potentially irritating ingredients. Sourcing truly uncoated, raw, and inert zinc oxide requires vetting suppliers for purity and transparency.
- Purity and Contaminants: Lower-grade zinc oxide may contain heavy metals (lead, arsenic, cadmium). Suppliers must provide up-to-date Certificates of Analysis (CoA) confirming compliance with strict limits (e.g., USP, EU cosmetic regulations).
2. Beef Tallow Sourcing and Quality Variability
The quality and safety of beef tallow significantly impact the final product’s stability, odor, and non-toxic profile.
- Source Animal Welfare and Diet: Tallow from conventionally raised, grain-fed cattle may contain higher levels of inflammatory fats and potential pesticide/hormone residues. Truly “non-toxic” tallow should come from pasture-raised, grass-fed, and grass-finished animals raised without antibiotics or hormones. Verifying this requires direct supplier relationships and traceability documentation.
- Processing Methods: Poor rendering practices (high heat, chemical solvents) can degrade tallow quality and create trans fats or off-odors. Sourcing tallow rendered slowly at low temperatures (traditional or wet rendering) preserves nutrients and reduces rancidity risk.
- Oxidative Stability and Rancidity: Tallow is prone to oxidation, leading to rancidity, unpleasant smells, and potentially irritating byproducts. Suppliers should provide peroxide value and anisidine value data, and tallow should be stored with antioxidants (e.g., vitamin E) and in dark, airtight containers.
3. Formulation and Stability Challenges
Combining mineral sunscreen with animal fats introduces formulation complexities.
- Dispersion and Whitening: Achieving even dispersion of zinc oxide in tallow without clumping is difficult. Poor dispersion leads to uneven UV protection and excessive whitening. This requires specialized equipment (e.g., homogenizers) and formulation expertise.
- Emulsion Stability (if applicable): If water-based ingredients (e.g., hydrosols, aloe) are added, creating a stable emulsion without synthetic emulsifiers is challenging. Separation over time or temperature changes can compromise efficacy and user experience.
- Microbial Growth: Natural products without broad-spectrum preservatives are prone to microbial contamination. Tallow-based sunscreens with any water content require effective, natural-compatible preservatives (e.g., radish root ferment, rosemary extract blends) validated through challenge testing.
4. Misleading Claims and Lack of Transparency (IP and Marketing)
The natural skincare market is rife with greenwashing, making it difficult to distinguish genuinely high-quality products.
- “Non-Toxic” Claims Without Verification: The term “non-toxic” is unregulated. Suppliers may use it without substantiating ingredient purity or safety. Demand full ingredient disclosure and third-party testing for contaminants.
- Intellectual Property (IP) Risks: Unique formulations combining tallow and zinc oxide may be protected by patents or trade secrets. Sourcing from a manufacturer using such IP without licensing could lead to legal disputes. Conduct due diligence on formulation originality and freedom to operate.
- Traceability Gaps: Without batch-specific traceability for both zinc oxide and tallow, verifying ethical sourcing and quality consistency across batches is impossible. Insist on documented supply chains and lot tracking.
5. Regulatory and Labeling Compliance
Ensuring compliance with regional regulations (e.g., FDA OTC monograph in the US, EU Cosmetics Regulation) is essential.
- Sunscreen as a Drug (US): In the U.S., sunscreens are regulated as over-the-counter (OTC) drugs. Using zinc oxide as the active ingredient requires adherence to FDA monograph standards, including final product testing for SPF and water resistance—challenging for small-batch, natural formulators.
- Labeling Accuracy: Mislabeling SPF, water resistance, or ingredient concentrations can lead to regulatory action and consumer harm. Third-party testing of the final product is crucial.
- “Natural” or “Organic” Certification: Claims like “organic” require certification (e.g., USDA Organic, COSMOS). Tallow can be certified organic if sourced from certified organic animals, but zinc oxide cannot. Misleading certifications erode trust.
Avoiding these pitfalls requires rigorous supplier vetting, investment in third-party testing, formulation expertise, and a commitment to transparency and regulatory compliance. Prioritizing traceability, purity, and scientific validation is essential for sourcing a truly safe and effective zinc oxide sunscreen with non-toxic beef tallow.

H2: Logistics & Compliance Guide for Zinc Oxide Sunscreen (Non-Toxic, Beef Tallow-Based)
1. Product Overview
Product Name: Non-Toxic Zinc Oxide Sunscreen with Beef Tallow
Active Ingredient: Non-Nano Zinc Oxide (typically 15–25%)
Base: Beef Tallow (rendered suet from grass-fed, pasture-raised cattle)
Claimed Attributes:
– Broad-spectrum UVA/UVB protection
– Non-toxic, reef-safe, biodegradable
– Free from parabens, phthalates, synthetic fragrances, and chemical UV filters
– Suitable for sensitive skin
2. Regulatory Classification & Jurisdiction
United States (FDA – Food and Drug Administration)
- Status: Over-the-Counter (OTC) Monograph Product (Sunscreen)
- Active Ingredient: Zinc Oxide is a GRASE (Generally Recognized as Safe and Effective) ingredient under the FDA’s sunscreen monograph at concentrations up to 25%.
- Non-Nano Requirement: FDA does not currently distinguish between nano and non-nano in final regulations, but labeling as “non-nano” must be substantiated.
- Labeling: Must comply with 21 CFR Part 352 (Sunscreen Drug Products for Over-the-Counter Human Use).
- Must include:
- Drug Facts Panel
- Active ingredient(s) and concentration
- SPF value (must be tested per FDA protocols)
- Directions for use
- Warnings (e.g., avoid eyes, discontinue if rash occurs)
- Waterproof claims require testing (e.g., 40 or 80 minutes)
European Union (EU – CPNP & EU Cosmetics Regulation 1223/2009)
- Status: Cosmetic Product (Sunscreen = “Product with Sun Protection”)
- Notification: Must be registered in the CPNP (Cosmetic Products Notification Portal).
- Zinc Oxide: Permitted under Annex VI, List of UV Filters, with maximum concentration of 25%.
- Nano-Materials: If zinc oxide is nano, it must be declared with “[nano]” in the INCI list and reported in the CPNP 6 months prior to market. Non-nano avoids this requirement.
- Labeling:
- INCI list required
- Must include name/address of Responsible Person (RP) in EU
- No drug claims (e.g., “prevents skin cancer”)
- SPF testing per ISO 24444
Canada (Health Canada – Natural Health Products Directorate & Cosmetic Regulations)
- Status: Cosmetic (if SPF ≤ 30 and only physical blockers) OR Natural Health Product (NHP) (if SPF > 30 or making health claims)
- Zinc Oxide: Permitted in cosmetics and NHPs
- Labeling: Must follow Cosmetic Regulations (C.R.C., c. 376) or Natural Health Products Regulations
- NHP License: Required if selling SPF > 30 or making therapeutic claims
- Bilingual Labeling: English and French required
Australia (TGA – Therapeutic Goods Administration)
- Status: Listed Medicine (ARTG Entry Required)
- Sunscreen with SPF ≥ 4: Regulated as therapeutic goods
- Application: Must be listed on the Australian Register of Therapeutic Goods (ARTG)
- Sponsor: Must have an Australian sponsor
- Labeling: Complies with TGA guidelines, includes AUST L number
3. Ingredient Sourcing & Compliance
Beef Tallow
- Source Requirements:
- Must be from BSE-free countries (e.g., Australia, New Zealand, parts of South America)
- Preferably grass-fed, pasture-raised, and certified humane or organic (for marketing and consumer trust)
- Processing:
- Rendered at food-grade or cosmetic-grade facilities
- Must be free of preservatives, additives, or contaminants (e.g., BHA/BHT)
- Documentation:
- Certificate of Analysis (CoA) for each batch
- Proof of country of origin
- Halal/Kosher certification (if claiming)
Zinc Oxide (Non-Nano)
- Specification:
- Particle size > 100 nm (confirmed via electron microscopy or DLS)
- Coated or uncoated (coatings like dimethicone or stearic acid must be declared)
- Safety: Must be non-inhalable (avoid powdered forms); safe in topical applications
- Documentation:
- CoA with particle size data
- Declaration of absence of nano form (for EU/Canada)
4. Manufacturing & GMP Compliance
- Facility Requirements:
- Must comply with Good Manufacturing Practices (GMP):
- US: cGMP for OTC drugs (21 CFR 211) if marketed as drug
- EU: ISO 22716 (Cosmetic GMP)
- Australia: PIC/S GMP (for TGA-listed products)
- Batch Records: Full traceability of raw materials and finished goods
- Microbial Testing: Ensure product meets limits per USP <61> or Ph. Eur. 5.1.4
- Stability Testing: 12–36 months (accelerated and real-time) to support shelf life
5. Packaging & Labeling Compliance
Primary Requirements (All Regions):
- Child-resistant packaging (if applicable)
- Batch number and expiry date
- Net weight (in metric and imperial)
- Manufacturer/packer details
Region-Specific:
- US: Drug Facts Panel, SPF claim backed by testing
- EU: CPNP number, Responsible Person, INCI list
- Canada: Bilingual labeling, DIN or NPN (if NHP)
- Australia: ARTG number, sponsor details
Claims to Avoid:
- “Chemical-free” (misleading)
- “100% natural” (unless fully substantiated)
- “Prevents skin cancer” (therapeutic claim; restricted)
6. Import/Export Logistics
Export from USA:
- FDA Facility Registration: Required for foreign export
- Prior Notice Submission: For shipments arriving in the US (if re-importing)
- Customs Documentation:
- Commercial Invoice
- Packing List
- Certificate of Free Sale (issued by FDA upon request)
- Certificate of Analysis (CoA)
- Non-Nano Declaration (for EU/Canada)
Import into EU:
- Customs Code: 3304.99 (Other beauty or make-up preparations)
- VAT: Apply local VAT rate (varies by country)
- RP Responsibility: EU-based Responsible Person handles compliance and vigilance reporting
Import into Canada:
- Customs Tariff: 3304.99.00.00
- BSF (Border Services Form): Required
- NHP License: If applicable, must be provided at customs
7. Environmental & Ethical Compliance
- Reef-Safe Claims:
- Must not contain oxybenzone, octinoxate, or nano-zinc (if claiming reef-safe)
- Supported by biodegradability testing (e.g., OECD 301)
- Sustainability:
- Tallow sourced as by-product (not primary driver of cattle farming)
- Packaging: Recyclable, biodegradable, or refillable (e.g., aluminum tins)
- Animal Testing:
- Prohibited in EU (Cosmetics Regulation)
- Leaping Bunny or PETA certification enhances marketability
8. Post-Market Surveillance & Incident Reporting
- Adverse Event Monitoring:
- Must report serious adverse events to regulatory bodies:
- US FDA: MedWatch (within 15 days)
- EU: Via RP to national authority (e.g., MHRA, ANSM)
- Canada: Adverse Reaction Reporting Portal
- Australia: TGA via CALENDEX
- Product Recalls:
- Have a recall plan in place
- Notify regulators promptly
9. Key Certifications (Recommended)
| Certification | Purpose |
|————-|——–|
| NSF/ANSI 305 | For personal care products with organic ingredients |
| Leaping Bunny | Cruelty-free claim |
| BioGro / ECOCERT | Natural/organic cosmetics (EU/AU) |
| USDA Certified Biobased | For bio-based content (e.g., tallow) |
| Non-GMO Project Verified | If ingredients are non-GMO |
10. Summary Checklist Before Launch
✅ Finalized formula with CoAs for all ingredients
✅ SPF and broad-spectrum testing completed (FDA or ISO method)
✅ GMP-compliant manufacturing facility
✅ Region-specific labeling and regulatory submissions (FDA, CPNP, TGA, etc.)
✅ Import/export documentation prepared
✅ Responsible Person (EU) or Sponsor (AU) appointed
✅ Adverse event reporting system in place
✅ Eco/ethical claims substantiated (reef-safe, non-toxic, etc.)
Note: Regulations evolve. Consult a regulatory affairs specialist or legal counsel before market entry, especially for cross-border sales.
H2 – Logistics & Compliance Guide v1.1
Last Updated: April 2024
Conclusion:
When sourcing a non-toxic sunscreen containing zinc oxide and beef tallow, it is essential to prioritize clean, transparent ingredients and ethical production practices. Zinc oxide, especially in non-nano, uncoated forms, offers safe and effective broad-spectrum sun protection without penetrating the skin or harming marine life. When combined with nourishing, pasture-raised beef tallow—rich in fat-soluble vitamins and skin-supportive fatty acids—this formulation provides both protection and restoration for the skin.
To ensure safety and quality, select products from brands that emphasize third-party testing, full ingredient disclosure, and sustainability. Look for certifications such as non-GMO, cruelty-free, and grass-fed to confirm the integrity of the beef tallow. Avoid sunscreens with synthetic fragrances, parabens, chemical UV filters, or nano particles to maintain a truly non-toxic profile.
Ultimately, a zinc oxide and beef tallow sunscreen represents a holistic approach to sun care—merging natural ingredients with modern safety standards to support both personal health and environmental wellness. With careful sourcing, this type of sunscreen can be a powerful part of a clean, effective skincare routine.