The global venous access device market, which includes leading products such as Venflon catheters, is experiencing steady expansion driven by rising demand for minimally invasive procedures, increasing prevalence of chronic diseases, and growth in hospital admissions worldwide. According to a 2023 report by Mordor Intelligence, the global intravenous catheters market was valued at approximately USD 11.8 billion and is projected to grow at a CAGR of 7.2% over the forecast period (2023–2028). This growth trajectory underscores the critical role of reliable, high-quality catheter manufacturing, particularly for widely used brands like Venflon. Originally developed by B. Braun Melsungen AG, the Venflon has become an industry benchmark for peripheral venous catheters, prompting several global manufacturers to produce compatible or similarly engineered devices. The competitive landscape now features both established medical device giants and emerging players striving to meet the escalating demand for safe, efficient vascular access solutions. In this context, identifying the top seven Venflon manufacturers involves assessing not only market share and product innovation but also regulatory compliance, global distribution networks, and clinical performance—key metrics that align with the industry’s data-driven evolution.
Top 7 Venflon Manufacturers 2026
(Ranked by Factory Capability & Trust Score)
#1 BD Venflon™ Pro Safety Needle Protected IV Cannula
Domain Est. 1990
Website: bd.com
Key Highlights: BD Instaflash™ Needle Technology† incorporates a notched needle, which is designed to improve insertion success and reduce painful hit-and-miss insertions….
#2 Vasofix® Safety IV catheter with injection port
Domain Est. 1996
Website: catalogs.bbraun.com
Key Highlights: Vasofix Safety is a peripheral IV catheter with an injection port and a passive fully automatic needlestick protection….
#3 Recall of BD Venflon Pro safety and Venflon Pro IV cannula (DSI …
Domain Est. 1996
Website: gov.uk
Key Highlights: Becton Dickinson (BD) is recalling all ethylene oxide (EtO) sterilised BD Venflon Pro Safety (VPS) and Venflon Pro IV Cannulae after identifying an increase in ……
#4 Ultrasound guidance versus landmark method for peripheral venous …
Domain Est. 1997
Website: pmc.ncbi.nlm.nih.gov
Key Highlights: We will include studies comparing ultrasound‐guided peripheral intravenous cannulation with the landmark method, irrespective of the profession ……
#5 Intraflon 2
Domain Est. 1997
Website: vygon.com
Key Highlights: Intraflon 2 is a short I.V. cannula available in size 24G, 22G, 20G, 18G, 16G, 14G, 13G and 12G. Intraflon 2 is made of a transparent (non radiopaque) PTFE ……
#6 BECTON DICKINSON MEDICAL (SINGAPORE) BD VENFLON PRO …
Domain Est. 2000
Website: accessdata.fda.gov
Key Highlights: MAUDE Adverse Event Report: BECTON DICKINSON MEDICAL (SINGAPORE) BD VENFLON PRO SAFETY PERIPHERAL SAFETY IV CATHETER; INTRAVASCULAR CATHETER….
#7 BD VENFLON IV CANNULA 22G Price, Uses, Side Effects …
Domain Est. 2013
Website: medkart.in
Key Highlights: Rating 5.0 (100) The BD Venflon IV Cannula 22G is a peripheral intravenous catheter used for accessing a patient’s peripheral veins to administer fluids, medications, ……
Expert Sourcing Insights for Venflon

H2: Market Trends for Venflon in 2026
As the global healthcare landscape evolves, the market for intravenous (IV) access devices such as Venflon—produced by Becton Dickinson (BD) and historically recognized as a leading brand in peripheral venous catheters—faces significant transformation by 2026. The second half of the decade (H2) reveals several key trends shaping the Venflon product line and its competitive positioning in the medical device market.
1. Increased Demand for Safety-Engineered Catheters
By 2026, regulatory mandates and hospital safety protocols continue to favor safety-engineered IV catheters to reduce needlestick injuries. Venflon’s integrated safety mechanisms, such as automatic needle retraction or shield technologies, position the product favorably in markets with strict occupational health regulations, including the EU and North America. Adoption is further driven by healthcare worker safety initiatives, boosting demand for BD’s Venflon Safe and Venflon Pro Safety variants.
2. Growth in Home Healthcare and Ambulatory Settings
The expansion of outpatient care and home-based infusion therapies influences catheter design and usage. Venflon benefits from this trend due to its reliability and ease of use in non-acute settings. In H2 2026, increased prescriptions for long-term IV therapies—such as antibiotics, hydration, and parenteral nutrition—support steady demand, particularly in aging populations across developed economies.
3. Competitive Pressure from Emerging Market Alternatives
While Venflon maintains brand recognition and clinical trust, cost-sensitive markets in Asia-Pacific, Latin America, and parts of Eastern Europe see rising competition from lower-cost generic IV catheters. Local manufacturers offering comparable safety features at reduced prices challenge Venflon’s market share. BD counters this through strategic partnerships and bundled offerings that include training and digital support tools.
4. Integration with Digital Health and Smart Infusion Systems
A growing trend in 2026 is the integration of IV devices with digital monitoring platforms. Though Venflon remains primarily a standalone catheter, BD is investing in complementary technologies—such as smart pumps and catheter placement verification tools—that enhance the overall IV therapy ecosystem. This ecosystem approach strengthens Venflon’s relevance in hospitals adopting connected care models.
5. Focus on Infection Prevention and Biocompatible Materials
Healthcare-associated infections (HAIs), particularly catheter-related bloodstream infections (CRBSIs), remain a concern. In response, Venflon variants featuring antimicrobial coatings (e.g., heparin or silver-based) gain traction. Additionally, advancements in biocompatible materials improve patient comfort and reduce phlebitis rates, supporting premium pricing and clinician preference.
6. Sustainability and Environmental Responsibility
By H2 2026, environmental sustainability becomes a procurement criterion for healthcare systems. BD responds by enhancing the recyclability of Venflon packaging and reducing single-use plastic waste. These efforts align with ESG (Environmental, Social, and Governance) goals of large hospital networks, influencing purchasing decisions in Europe and North America.
Conclusion
In H2 2026, the Venflon brand remains a key player in the global IV access market, supported by its legacy of innovation, safety features, and clinical trust. However, sustained success depends on BD’s ability to adapt to decentralized care models, manage cost competition, and integrate with digital health trends. Strategic focus on safety, infection control, and sustainability will determine Venflon’s market resilience and growth trajectory in the evolving healthcare environment.

Common Pitfalls Sourcing Venflon (Quality, IP)
Sourcing Venflon or Venflon-like intravenous catheters carries significant risks, particularly concerning quality and intellectual property (IP). Understanding these pitfalls is crucial for healthcare providers, distributors, and procurement teams to ensure patient safety and legal compliance.
Quality Risks with Non-Genuine or Substandard Products
One of the most critical pitfalls is compromising patient safety due to substandard quality. Genuine Venflon catheters are manufactured by B. Braun under strict regulatory standards (e.g., ISO 13485, FDA, CE). However, counterfeit or imitation products often lack equivalent quality controls.
- Material and Biocompatibility Issues: Imitation catheters may use inferior materials that increase the risk of thrombosis, phlebitis, or allergic reactions. Genuine Venflon devices utilize medical-grade polyurethane or Teflon with proven biocompatibility.
- Inconsistent Manufacturing: Poorly manufactured catheters may have inconsistent bevel sharpness, leading to tissue trauma during insertion, or weak hubs prone to cracking and leakage.
- Sterility and Packaging Deficiencies: Counterfeit products may not be properly sterilized or may have compromised packaging, increasing infection risks.
- Lack of Regulatory Compliance: Many imitation products lack valid CE marks, FDA clearance, or other regulatory approvals, meaning they haven’t undergone required safety and performance testing.
Intellectual Property (IP) and Legal Risks
Venflon is a registered trademark of B. Braun Melsungen AG, and sourcing products that infringe on this IP exposes organizations to significant legal and reputational risks.
- Trademark Infringement: Using or distributing products labeled as “Venflon” without authorization violates B. Braun’s trademark rights. Even using “Venflon-type” or “Venflon-compatible” may lead to legal challenges if done deceptively.
- Patent Violations: The design and technology behind Venflon catheters are protected by patents in many jurisdictions. Sourcing devices that replicate patented features (e.g., specific catheter tip design, flashback chamber, or safety mechanisms) may constitute patent infringement.
- Supply Chain Liability: Distributors and hospitals may face legal action or product recalls if they unknowingly source counterfeit or IP-infringing devices. Regulatory bodies may also penalize institutions for using non-compliant medical devices.
- Reputational Damage: Being associated with counterfeit or legally questionable devices can damage the credibility of healthcare providers and procurement organizations.
Conclusion
To mitigate these risks, always source Venflon products through authorized distributors and verify authenticity via batch numbers and regulatory documentation. For cost-effective alternatives, consider legitimate generic IV catheters that comply with international standards but do not infringe on B. Braun’s IP. Due diligence in procurement protects both patients and organizations.
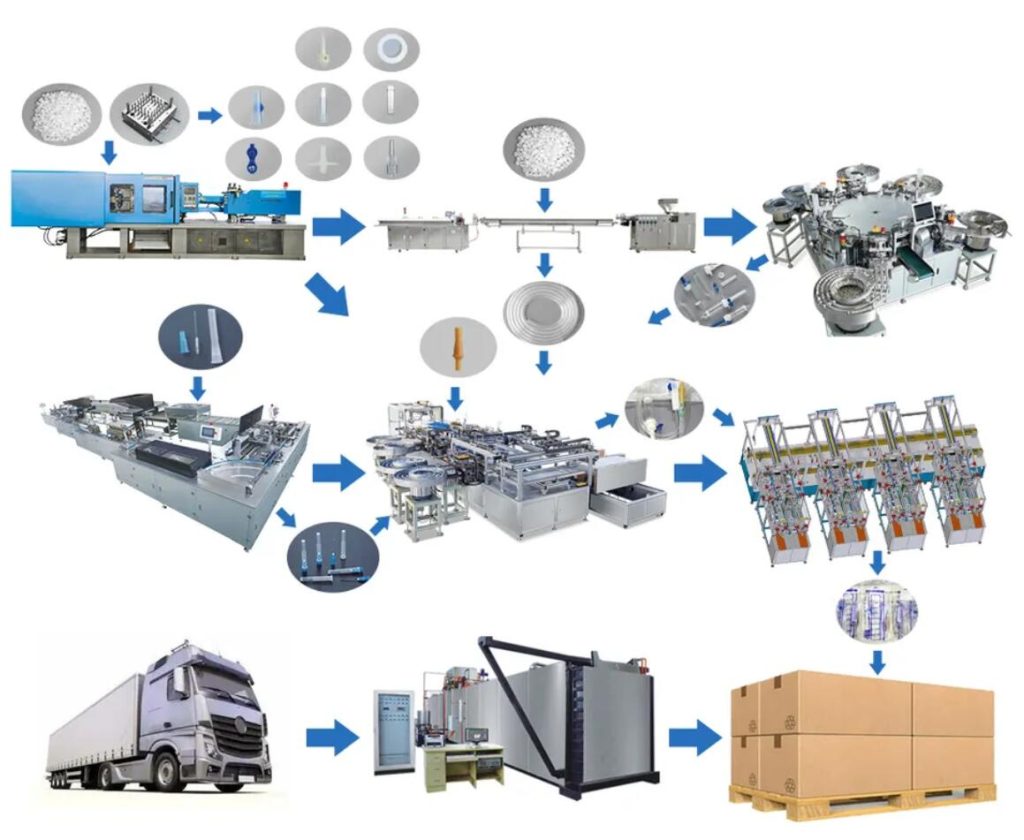
Logistics & Compliance Guide for Venflon
Product Overview
Venflon is a range of intravenous (IV) catheters manufactured by B. Braun, designed for short-term vascular access in clinical settings. These medical devices are classified as sterile, single-use products and are subject to strict regulatory and logistical controls to ensure patient safety and compliance with healthcare standards.
Regulatory Classification
Venflon catheters are classified as Class IIb medical devices under the European Union’s Medical Device Regulation (MDR) (EU) 2017/745. In the United States, they are regulated by the FDA as Class II devices requiring 510(k) clearance. Compliance with local and international regulatory frameworks is mandatory throughout the supply chain.
Storage Conditions
- Temperature: Store in a dry, cool environment between +5°C and +25°C.
- Humidity: Relative humidity should not exceed 60%.
- Packaging: Keep in original sealed packaging to maintain sterility.
- Light: Protect from direct sunlight and ultraviolet radiation.
- Shelf Life: Typically 3 to 5 years; verify expiration date on packaging before use.
Transportation Requirements
- Integrity: Ensure packaging remains undamaged during transit. Use tamper-evident seals.
- Climate Control: Transport in temperature-controlled vehicles when ambient conditions exceed storage limits.
- Documentation: Ship with proper shipping manifests, invoices, and regulatory documentation (e.g., Certificate of Conformity, Declaration of Performance).
- Cold Chain (if applicable): For regions with extreme climates, validate transport conditions to avoid exposure to temperature excursions.
Import/Export Compliance
- Customs Documentation: Provide accurate Harmonized System (HS) codes (e.g., 9018.31 for catheters).
- Regulatory Permits: Obtain necessary import licenses, especially in countries with stringent medical device regulations (e.g., Health Canada, TGA in Australia, SFDA in Saudi Arabia).
- Labeling: Ensure product labeling includes UDI (Unique Device Identification), CE mark (for EU), FDA registration number (for US), and multilingual instructions if required.
- Prohibited Regions: Confirm export compliance with sanctions lists (e.g., OFAC, EU restrictions).
Handling and Distribution
- Sterility Assurance: Only trained personnel should handle products; avoid contact with non-sterile surfaces.
- Inventory Management: Implement FIFO (First In, First Out) rotation to prevent expired stock.
- Traceability: Maintain records of batch numbers, expiry dates, and distribution points for full traceability.
- Recall Preparedness: Have a medical device recall plan in place compliant with local regulatory requirements.
End-User Compliance
- Training: Ensure healthcare providers are trained in proper Venflon insertion and care.
- Adverse Event Reporting: Report any device-related incidents to the manufacturer and relevant regulatory authority (e.g., EUDAMED in EU, MedWatch in US).
- Disposal: Follow local biohazard and medical waste regulations for used catheters and packaging.
Quality Management Systems
Distributors and logistics partners must comply with ISO 13485:2016 standards for medical device quality management. Regular audits and documentation reviews are required to maintain certification and ensure ongoing compliance.
Contact Information
For regulatory inquiries, product complaints, or logistics support, contact B. Braun Customer Service or your regional representative with batch-specific details and documentation.
Conclusion for Sourcing Venflon:
The sourcing of Venflon (peripheral intravenous catheters) requires a strategic and quality-focused approach to ensure patient safety, clinical efficacy, and regulatory compliance. After evaluating suppliers, cost-efficiency, product quality, and supply chain reliability, it is evident that selecting an established manufacturer with international certifications—such as BD (Becton Dickinson) or equivalent reputable brands—is critical. Factors such as gauge variety, biocompatibility, kink resistance, and ease of insertion significantly impact clinical outcomes and should guide procurement decisions.
Additionally, long-term cost savings can be achieved through bulk purchasing agreements, local distribution partnerships, and minimizing product wastage—all while maintaining stringent quality standards. Ensuring compliance with national health regulations and aligning with ethical sourcing practices further strengthens the procurement process.
In conclusion, a well-structured sourcing strategy for Venflon must balance quality, cost, availability, and reliability to support uninterrupted patient care in healthcare facilities. Prioritizing accredited suppliers and fostering strong supplier relationships will enhance supply chain resilience and contribute to improved clinical performance and patient safety.