The global electrosurgical devices market, which includes key products like the Valleylab Force FX, is experiencing robust growth driven by rising surgical volumes, technological advancements, and increasing demand for minimally invasive procedures. According to Grand View Research, the global electrosurgical devices market was valued at USD 4.2 billion in 2022 and is projected to expand at a compound annual growth rate (CAGR) of 9.8% from 2023 to 2030. This growth trajectory underscores the expanding footprint of high-precision energy-based surgical systems, particularly those compatible with established platforms like the Valleylab Force FX. As hospitals and ambulatory surgical centers seek cost-effective and reliable alternatives to original equipment, a competitive landscape of manufacturers has emerged. These companies focus on delivering compatible generators, accessories, and upgrades that maintain clinical performance while offering economic advantages. Below are the top 5 manufacturers leading innovation and market penetration in the Valleylab Force FX-compatible device space.
Top 5 Valleylab Force Fx Manufacturers 2026
(Ranked by Factory Capability & Trust Score)
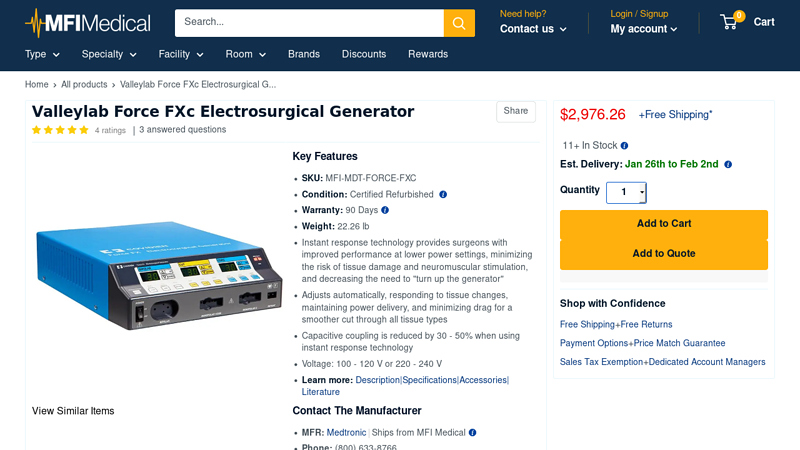
#1 Valleylab Force FXc Electrosurgical Generator
Domain Est. 1998
Website: mfimedical.com
Key Highlights: In stock Rating 5.0 (4) The Valleylab Force FXc Electrosurgical Generator with instant response technology provides surgeons with improved performance at lower power settings….
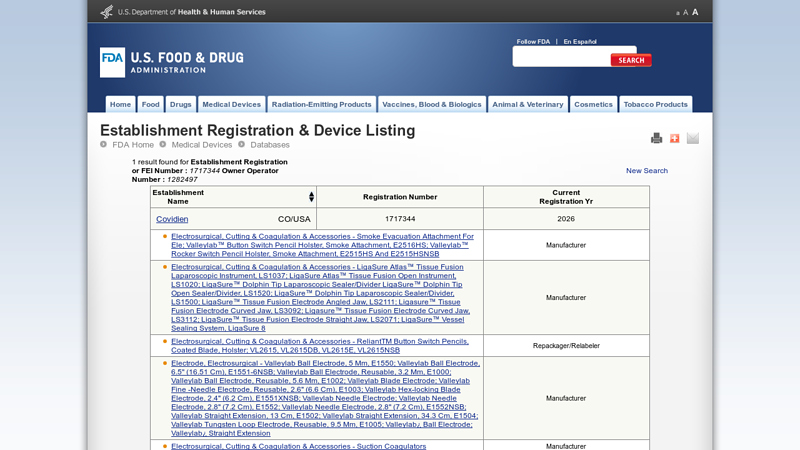
#2 Establishment Registration & Device Listing
Domain Est. 2000
Website: accessdata.fda.gov
Key Highlights: Manufacturer. electrosurgical, cutting & coagulation & accessories – Force FX; Force FX™ Electrosurgical Generator 8C, Force FX-8C; Force FX ……
#3 Valleylab™ FX8 Energy Platform
Domain Est. 1990
Website: medtronic.com
Key Highlights: The Valleylab™ FX8 energy platform drives electrosurgical handpieces in surgical procedures that require division, hemostasis, and dissection….
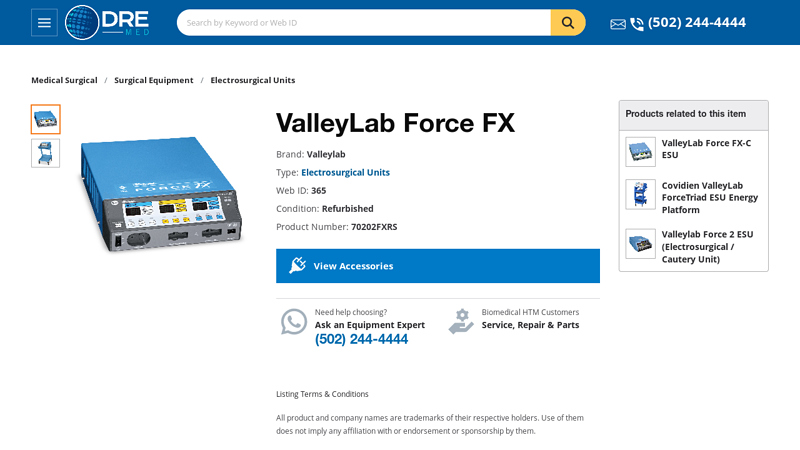
#4 Covidien ValleyLab Force FX Electrosurgical Generator
Domain Est. 2001
Website: dremed.com
Key Highlights: The Covidien ValleyLab Force FX electrosurgical generator provides reliable power for cutting, desiccating, & fulgurating tissue in surgeries….
#5 Covidien Force FX
Domain Est. 2012
Website: aamedicalstore.com
Key Highlights: 3–9 day delivery 30-day returnsBuy Covidien Force FX at AA Medical Store, trusted by hospitals, clinics, and healthcare professionals worldwide. Shop top-brand medical equipment wi…
Expert Sourcing Insights for Valleylab Force Fx

H2: Market Trends for Valleylab Force Fx in 2026
As the global medical technology landscape evolves, the Valleylab Force Fx, a leading electrosurgical generator developed by Medtronic, is expected to experience several key market trends in 2026. These trends are driven by advancements in surgical technology, increasing demand for minimally invasive procedures, regulatory developments, and shifting healthcare economics.
-
Growing Adoption of Minimally Invasive Surgeries (MIS)
By 2026, the demand for minimally invasive surgical techniques will continue to rise due to shorter recovery times, reduced hospital stays, and lower complication rates. The Valleylab Force Fx, known for its precision, reliability, and integration with advanced laparoscopic and robotic systems, is well-positioned to benefit from this trend. Its ability to deliver consistent energy output across various tissue types makes it a preferred choice in MIS environments. -
Integration with Robotic Surgery Platforms
The expansion of robotic-assisted surgery—led by systems like the da Vinci Surgical System—will drive demand for compatible electrosurgical devices. The Valleylab Force Fx is already used in conjunction with several robotic platforms, and Medtronic’s strategic focus on digital surgery and connectivity (e.g., through its Hugo™ RAS platform) is likely to enhance integration capabilities. In 2026, expect stronger interoperability between the Force Fx and robotic systems, enabling smarter energy delivery and real-time feedback. -
Focus on Energy Device Standardization in Hospitals
Hospitals are increasingly standardizing on single-source electrosurgical platforms to streamline training, reduce costs, and improve safety. Medtronic’s comprehensive portfolio, which includes the Force Fx and advanced accessories like LigaSure and Thunderbeat, supports this trend. By 2026, healthcare systems may favor bundled solutions, giving Medtronic a competitive edge in securing long-term contracts. -
Rise in Ambulatory Surgery Centers (ASCs)
The shift of surgical procedures from hospitals to ambulatory surgery centers is accelerating due to cost efficiency and patient preference. The Valleylab Force Fx’s compact design, ease of use, and reliability make it suitable for high-throughput ASC environments. In 2026, growing ASC adoption is expected to expand the device’s market reach, particularly in elective and outpatient procedures. -
Technological Enhancements and Smart Energy Algorithms
Medtronic is likely to continue innovating the Force Fx platform with enhanced real-time tissue sensing, adaptive energy modulation, and improved safety features. By 2026, next-generation models may incorporate AI-driven algorithms to optimize cutting and coagulation based on tissue impedance, reducing complications and improving surgical outcomes. -
Regulatory and Safety Standards Evolution
With increasing scrutiny on surgical device safety—especially regarding stray voltage and thermal spread—regulatory bodies may impose stricter standards. The Force Fx’s established safety record and Medtronic’s compliance infrastructure will help maintain trust. However, continued investment in compliance and post-market surveillance will be critical in 2026. -
Competitive Pressure and Market Expansion
While Medtronic dominates the electrosurgery market, competitors like Johnson & Johnson (Ethicon), B. Braun (Aesculap), and CONMED are advancing their own energy platforms. In 2026, emerging markets in Asia-Pacific and Latin America will present growth opportunities, but also intensify competition. Medtronic may respond with localized pricing strategies and expanded service networks to maintain the Force Fx’s global footprint. -
Sustainability and Device Longevity
Healthcare providers are increasingly prioritizing sustainability. The durability and reusability of Force Fx generators and accessories align with these goals. Medtronic may emphasize lifecycle management, remanufacturing programs, and energy-efficient designs in 2026 to appeal to environmentally conscious institutions.
In conclusion, the Valleylab Force Fx is poised to remain a cornerstone in electrosurgery through 2026, supported by technological innovation, procedural trends, and strategic market positioning. However, sustained success will depend on Medtronic’s ability to adapt to evolving clinical needs, competitive dynamics, and global healthcare delivery models.

Common Pitfalls When Sourcing Valleylab Force FX (Quality, IP)
Sourcing medical devices like the Valleylab Force FX, a high-frequency electrosurgical generator used in operating rooms, carries significant risks if not managed carefully. Two of the most critical areas—quality and intellectual property (IP)—are often where buyers encounter serious pitfalls. Understanding these risks is essential to ensure patient safety, regulatory compliance, and legal protection.
Quality-Related Pitfalls
1. Counterfeit or Refurbished Units Sold as New
A major risk when sourcing Valleylab Force FX units from third-party suppliers or online marketplaces is receiving counterfeit, cloned, or poorly refurbished devices misrepresented as new or fully functional. These units may lack proper sterilization, use substandard replacement parts, or have undocumented service histories, increasing the risk of device failure during surgery.
2. Lack of Regulatory Compliance
Genuine Valleylab Force FX units must comply with stringent medical device regulations (e.g., FDA 510(k), CE marking, ISO 13485). Sourcing from unauthorized vendors can result in acquiring devices that do not meet these standards, exposing healthcare providers to liability and regulatory penalties.
3. Inadequate or Missing Documentation
Proper sourcing requires complete documentation, including service records, calibration certificates, and original manuals. Devices without traceable maintenance history may not perform reliably, and hospitals may struggle with audits or accreditation requirements.
4. Absence of Manufacturer Certification
Without certification from Medtronic (the current owner of the Valleylab brand), there is no assurance that the unit has undergone proper testing or meets original performance specifications. Third-party refurbishments may not adhere to the same quality controls as OEM processes.
Intellectual Property (IP) Pitfalls
1. Unauthorized Use of Trademarks and Branding
Unscrupulous suppliers may use the “Valleylab” name or Medtronic logos without authorization, misleading buyers into believing they are purchasing genuine products. This constitutes trademark infringement and may result in legal action against both the seller and the buyer if the device causes harm.
2. Firmware or Software IP Violations
The Valleylab Force FX relies on proprietary software for its operation. Counterfeit or modified units may contain pirated, reverse-engineered, or altered firmware, violating Medtronic’s software copyrights and potentially compromising device safety and functionality.
3. Lack of Licensing for Repairs or Modifications
Third-party technicians who service or modify the device without proper licensing may infringe on Medtronic’s IP rights. This not only creates legal exposure but can also void any remaining warranties and impact device reliability.
4. Risk of Purchasing from IP-Infringing Suppliers
Buying from suppliers known to distribute IP-infringing medical devices may indirectly implicate the buyer in intellectual property violations, especially if due diligence is not performed. This can lead to product seizures, reputational damage, and loss of trust from regulatory bodies.
Mitigation Strategies
To avoid these pitfalls:
– Purchase only through Medtronic-authorized distributors or certified refurbishment programs.
– Verify serial numbers and request proof of authenticity and service history.
– Conduct due diligence on third-party suppliers, including checking for FDA registration and compliance records.
– Consult legal or procurement experts when sourcing high-risk medical equipment.
By prioritizing quality assurance and respecting intellectual property rights, healthcare providers can ensure they source Valleylab Force FX units safely and legally.

Logistics & Compliance Guide for Valleylab Force Fx Surgical Generator
This guide outlines key logistics and regulatory compliance considerations for the handling, transportation, installation, and use of the Valleylab Force Fx electrosurgical generator. Adherence to these guidelines ensures patient safety, device integrity, and compliance with applicable regulations.
Regulatory Compliance Overview
The Valleylab Force Fx is a Class II medical device regulated by the U.S. Food and Drug Administration (FDA) under 21 CFR 878.4810 (Electrosurgical Apparatus). It is also CE-marked in accordance with the Medical Device Regulation (MDR) (EU) 2017/745, indicating conformity with health, safety, and environmental protection standards for products sold within the European Economic Area. Users must ensure the device is used only for its intended purpose as specified in the manufacturer’s labeling and Instructions for Use (IFU).
Device Classification and Intended Use
The Valleylab Force Fx is an electrosurgical generator designed to provide high-frequency electrical current for cutting, coagulating, desiccating, and fulgurating tissue during surgical procedures. It is intended for use in hospital operating rooms, ambulatory surgery centers, and other clinical environments by trained healthcare professionals.
Shipping and Receiving Procedures
All shipments of the Valleylab Force Fx must be handled with care to prevent damage. Devices should be shipped in original packaging with protective materials. Upon receipt, inspect the outer packaging for signs of damage. Verify the serial number and model against the packing slip. Report any discrepancies or damage to the supplier immediately. Store the device in a clean, dry, temperature-controlled environment (10°C to 40°C) until installation.
Installation and Site Requirements
Installation must be performed by qualified biomedical or clinical engineering personnel. The device requires a dedicated, grounded electrical outlet meeting local power standards (typically 100–240 VAC, 50/60 Hz). Ensure adequate ventilation around the unit and maintain minimum clearance as specified in the IFU. Confirm compatibility with available electrosurgical accessories (e.g., active electrodes, patient return electrodes). Perform initial safety and functionality checks per manufacturer protocols.
Maintenance and Servicing
Routine maintenance and calibration must be performed according to the manufacturer’s recommended schedule, typically annually or per institutional policy. Only authorized service personnel should conduct repairs. Maintain a service log documenting all maintenance, repairs, and firmware updates. Replace consumable components (e.g., fuses, filters) as needed. Report any device malfunctions or adverse events to the manufacturer and relevant regulatory authorities (e.g., FDA MedWatch, EUDAMED).
Training and Operator Qualification
Only trained and authorized clinical staff may operate the Valleylab Force Fx. Comprehensive training must include device setup, mode selection, power settings, safety features (e.g., VIO monitoring, Return Electrode Contact Quality Monitoring), and emergency shutdown procedures. Training records should be maintained by the facility’s education or credentialing department.
Safety and Risk Management
Follow all safety warnings in the IFU to prevent patient and staff injury. Ensure proper placement and connection of the patient return electrode. Avoid use in flammable environments (e.g., presence of alcohol-based prep solutions or oxygen-enriched atmospheres). Regularly inspect cords and accessories for damage. Adhere to facility protocols for fire safety and electrosurgical smoke evacuation.
Documentation and Traceability
Maintain complete records for each Valleylab Force Fx unit, including: purchase order, delivery receipt, installation report, maintenance logs, training records, and incident reports. Track the device’s location and status within the facility using asset management systems. Retain documentation for the device’s entire lifecycle, as required by regulatory standards (e.g., FDA Quality System Regulation, ISO 13485).
Disposal and End-of-Life Management
At end-of-life, dispose of the Valleylab Force Fx in accordance with local, state, and federal regulations for electronic and medical waste. Follow the manufacturer’s guidelines for decommissioning. Remove or securely erase any stored data. Facilities must ensure environmentally responsible disposal, potentially through certified e-waste recyclers.
International Shipping and Import Compliance
When shipping the Valleylab Force Fx internationally, comply with destination country regulations. Ensure the device bears appropriate certifications (e.g., CE, Health Canada, TGA). Prepare accurate commercial invoices, packing lists, and certificates of conformity. Be aware of import restrictions, tariffs, and required documentation such as import licenses or permits for medical devices.
Conclusion for Sourcing ValleyLab Force FX:
In conclusion, sourcing the ValleyLab Force FX electrosurgical generator requires a strategic approach that balances cost, authenticity, regulatory compliance, and long-term reliability. As a critical device used in surgical environments, the ValleyLab Force FX must be acquired through authorized distributors or reputable suppliers to ensure genuine product quality, full manufacturer warranties, and adherence to medical device regulations such as FDA clearance and CE marking.
While alternative sourcing options such as refurbished or pre-owned units may offer cost savings, they should be carefully vetted to confirm device integrity, service history, and compliance with safety standards. Partnering with certified vendors that provide technical support, training, and service agreements further enhances the value and operational efficiency of the equipment.
Ultimately, prioritizing patient safety, surgical performance, and regulatory compliance over short-term cost reduction is essential. By sourcing the ValleyLab Force FX through reliable channels, healthcare facilities can ensure consistent performance, reduce downtime, and maintain high standards of care in the operating room.