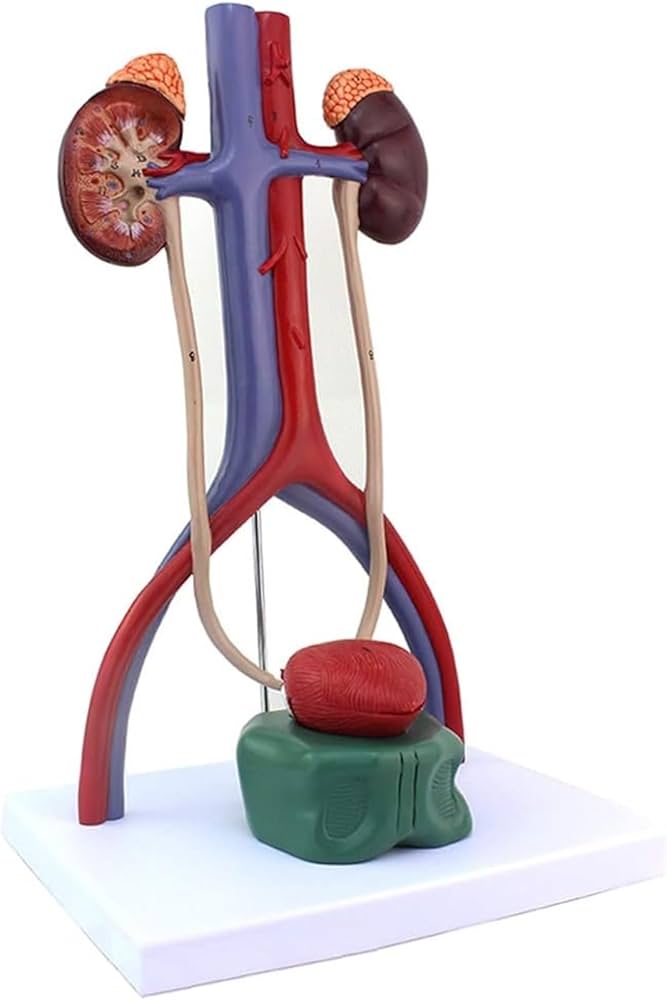
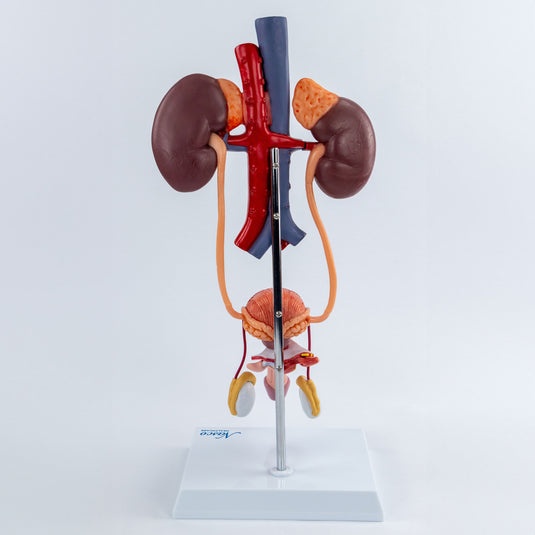
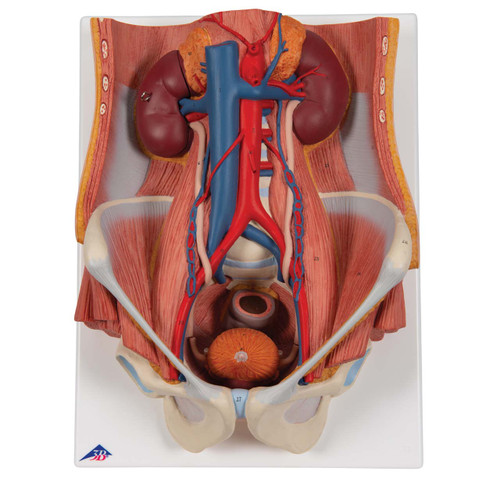
The global urology devices market is experiencing robust growth, driven by rising prevalence of urinary tract disorders, increasing geriatric population, and advancements in diagnostic and therapeutic technologies. According to Mordor Intelligence, the market was valued at USD 28.6 billion in 2023 and is projected to grow at a CAGR of 6.8% from 2024 to 2029. This expansion underscores the growing demand for accurate, reliable, and anatomically precise urinary models used in medical training, surgical planning, and patient education. As healthcare institutions and educational facilities prioritize hands-on simulation and visual learning tools, manufacturers of urinary models are scaling innovation to meet evolving clinical needs. Based on product quality, technological integration, market presence, and customer reviews, the following eight companies have emerged as leaders in the design and production of high-fidelity urinary system models.
Top 8 Urinary Model Manufacturers 2026
(Ranked by Factory Capability & Trust Score)
#1 Products
Domain Est. 1990
Website: bd.com
Key Highlights: Medical Device Manufacturer. B2B and Industry Partners. Laboratory. Antimicrobial … Site-Rite Halcyon™7. Site-Rite™34. Site-Scrub™2. SkyLite™2. SlimPort™10….
#2 Urinary Bladder Matrix (UBM) Technology
Domain Est. 1999
Website: marketing.integralife.com
Key Highlights: The ACell portfolio, acquired by Integra LifeSciences, features Urinary Bladder Matrix (UBM), the technology platform of Cytal, MicroMatrix and Gentrix….
#3 Urinary Models
Domain Est. 1999
Website: universalmedicalinc.com
Key Highlights: 1–7 day delivery 14-day returnsVisit our complete line of urinary models which range from basic patient education models to detailed urinary models that are well-suited for med stu…
#4 0145
Domain Est. 1999
#5 Somso Human Urinary System Model
Domain Est. 2006
Website: norecopa.no
Key Highlights: Shows major components of the urinary system: kidney, renal artery, vein, ureter, bladder and urethra. Life Size. Somso model….
#6 Urology Models
Domain Est. 2011
#7 Ethicon Surgical Technologies
Domain Est. 2021
Website: jnjmedtech.com
Key Highlights: Discover Ethicon’s surgical technologies, designed to support care teams and help improve patient outcomes, backed by decades of expertise and quality….
#8 Urinary Organs › Anatomy models
Website: somso.de
Key Highlights: Natural size, in SOMSO-PLAST®, Kidneys, ureters, adrenal glands and bladder with prostate, as well as the large abdominal vessels shown….
Expert Sourcing Insights for Urinary Model
H2: 2026 Market Trends for the Urinary Model Sector
The urinary model market—a segment within medical simulation and urology training—is poised for significant evolution by 2026, driven by advancements in medical education, technological innovation, and rising demand for precision in urological procedures. These models, used for training healthcare professionals in catheterization, cystoscopy, and other urological interventions, are increasingly incorporating smart technologies and realistic anatomical design. Below are the key trends expected to shape the urinary model market in 2026:
-
Integration of Augmented and Virtual Reality (AR/VR)
By 2026, AR- and VR-enhanced urinary models are expected to become mainstream in clinical training programs. These immersive platforms allow trainees to practice procedures in simulated real-time environments with haptic feedback, increasing skill acquisition and confidence. Institutions are investing in hybrid models that combine physical simulators with digital overlays to provide real-time performance analytics. -
Growth in Demand for High-Fidelity Simulators
The shift toward competency-based medical education is fueling demand for high-fidelity urinary models that replicate human tissue texture, anatomical variations, and physiological responses. Manufacturers are focusing on developing gender-specific and pathology-inclusive models (e.g., for benign prostatic hyperplasia or urethral strictures) to better prepare clinicians for real-world scenarios. -
Expansion in Emerging Markets
With increasing healthcare infrastructure development in Asia-Pacific, Latin America, and parts of Africa, there is growing adoption of medical simulation tools. By 2026, these regions are expected to represent a significant portion of market growth, supported by government initiatives to improve medical training standards and reduce clinical errors. -
Rise of AI-Powered Feedback Systems
Urinary models integrated with artificial intelligence are enabling real-time assessment of user technique. AI algorithms analyze procedural accuracy, hand movements, and decision-making, providing personalized feedback. This trend is enhancing training efficiency and standardization across institutions. -
Focus on Reusable and Sustainable Designs
Environmental and cost concerns are pushing manufacturers toward durable, sterilizable, and modular urinary models. In 2026, sustainability will be a key purchasing criterion, with institutions favoring models that reduce single-use plastics and support long-term reuse without compromising realism. -
Increased Collaboration Between Industry and Academia
Strategic partnerships between medical device companies and academic medical centers are accelerating product innovation. These collaborations facilitate user-centered design, ensuring that urinary models meet the practical needs of educators and trainees, ultimately improving clinical outcomes. -
Regulatory and Standardization Developments
As simulation becomes integral to certification processes, regulatory bodies are expected to establish clearer guidelines for simulation-based training. By 2026, standardized performance benchmarks for urinary model use may be adopted globally, influencing procurement and training protocols.
In conclusion, the urinary model market in 2026 will be defined by technological sophistication, global accessibility, and alignment with modern educational standards. Stakeholders—ranging from manufacturers to medical educators—must adapt to these trends to remain competitive and effective in advancing urological care.

Common Pitfalls Sourcing Urinary Models: Quality and Intellectual Property (IP) Concerns
Sourcing urinary models—whether for medical training, research, or commercial product development—presents several potential pitfalls, particularly concerning product quality and intellectual property rights. Overlooking these issues can lead to compromised performance, legal disputes, and reputational damage. Below are the key challenges to consider.
Quality-Related Pitfalls
Inconsistent Material Fidelity
Many urinary models use synthetic materials that may not accurately replicate the texture, elasticity, or anatomical response of real human tissue. Low-quality models often fail to simulate realistic catheter insertion, urethral resistance, or bladder compliance, undermining their training or testing value.
Poor Anatomical Accuracy
Inaccurate proportions, misaligned internal structures (e.g., prostate enlargement, urethral strictures), or oversimplified designs can reduce the model’s clinical relevance. This is especially critical in pathology-specific models used for surgical training or device testing.
Durability and Reusability Issues
Low-cost models may degrade quickly after repeated use, leading to tearing, leakage, or loss of structural integrity. This increases long-term costs and creates inconsistencies in training or testing environments.
Lack of Standardization and Validation
Many commercially available models lack independent validation or adherence to industry standards (e.g., ISO, ASTM). Without third-party testing or peer-reviewed data supporting their performance, it’s difficult to assess reliability.
Intellectual Property (IP)-Related Pitfalls
Unlicensed Use of Protected Designs
Some suppliers may replicate patented urinary model designs without authorization. Sourcing from such vendors exposes the buyer to legal risks, including contributory infringement claims, especially if the model is used commercially.
Ambiguous or Missing IP Documentation
Suppliers may fail to provide clear licensing agreements, copyright notices, or patent disclaimers. This opacity makes it difficult to determine whether modifications, reproductions, or commercial use of the model are permitted.
Risk of Reverse Engineering Violations
When customizing or improving a sourced model, there’s a risk of inadvertently infringing on existing IP if the original design contains protected elements. Without proper due diligence, such actions could lead to litigation.
Unclear Ownership in Custom Development
If working with a manufacturer to develop a custom urinary model, contracts that don’t explicitly assign IP rights to the buyer can result in shared or lost ownership. This limits control over future production, distribution, and monetization.
Mitigation Strategies
To avoid these pitfalls, organizations should:
– Conduct thorough due diligence on suppliers, including requesting validation studies and quality certifications.
– Require clear IP disclosures and written warranties from vendors.
– Engage legal counsel to review licensing terms and custom development agreements.
– Prioritize suppliers with transparent manufacturing processes and a history of regulatory compliance.
By addressing quality and IP concerns proactively, stakeholders can ensure they procure reliable, legally sound urinary models that meet their operational and ethical standards.
Logistics & Compliance Guide for Urinary Model
This guide outlines the essential logistics and compliance requirements for the handling, transportation, storage, and regulatory adherence related to the Urinary Model medical device.
Product Overview and Classification
The Urinary Model is a Class II medical device intended for diagnostic and monitoring use in urological applications. It is regulated under FDA 21 CFR Part 876 and EU MDR Annex VIII, Class IIa. The device must be distributed and used in accordance with regional regulatory standards.
Regulatory Compliance
All shipments and distributions must comply with applicable regulations, including FDA (U.S.), Health Canada (Canada), EU MDR (European Union), and other local health authority requirements. Documentation must include a valid Certificate of Conformity, Declaration of Medical Device Conformity (DoC), and labeling in the local language where required.
Labeling and Packaging Requirements
Each unit must be labeled with the UDI (Unique Device Identifier), lot number, expiration date, and manufacturer details. Packaging must be sterile, tamper-evident, and compliant with ISO 11607 for medical device packaging. Outer shipping containers must display proper medical device labeling, handling symbols, and hazard warnings if applicable.
Storage Conditions
The Urinary Model must be stored in a dry, climate-controlled environment between 15°C and 30°C (59°F to 86°F) with relative humidity below 60%. Avoid exposure to direct sunlight, radiation, or extreme temperatures. Devices must remain sealed until point of use to maintain sterility.
Transportation Guidelines
Transport via certified medical device logistics providers only. Use validated cold chain solutions if required. All shipments must include temperature monitoring for traceability. International shipments must comply with IATA Dangerous Goods Regulations if batteries or other regulated components are included.
Import/Export Documentation
For cross-border movement, provide commercial invoice, packing list, bill of lading, and regulatory export documentation (e.g., U.S. FDA Export Certificate, EU Statement of Conformity). Ensure compliance with import regulations in the destination country, including potential need for local representation or import licenses.
Inventory and Traceability
Maintain full traceability through UDI and lot tracking. Implement a robust inventory management system that supports recalls and field safety corrective actions (FSCAs). Records must be retained for a minimum of 10 years post-device expiration.
Recall and Post-Market Surveillance
Establish a recall procedure aligned with FDA 21 CFR Part 806 and EU MDR Article 87. Report adverse events and field safety notices promptly to regulatory bodies. Conduct regular post-market surveillance to ensure ongoing compliance and device safety.
Training and Documentation
Personnel involved in logistics and handling must be trained on medical device regulations, GCP, and internal SOPs. Training records must be maintained and updated annually.
Environmental and Disposal Compliance
Dispose of non-conforming or expired units in accordance with local biomedical waste regulations. Do not discard in general waste streams. Partner with certified medical waste disposal services.
Conclusion for Sourcing Urinary Model:
In conclusion, sourcing a high-quality urinary model requires careful evaluation of several key factors, including anatomical accuracy, durability, material safety, educational functionality, and cost-effectiveness. Whether intended for medical training, patient education, or academic purposes, selecting a model that closely replicates human urinary system anatomy enhances learning outcomes and practical application. Additionally, considering supplier reputation, customization options, and after-sales support ensures long-term satisfaction and utility. By prioritizing these aspects, institutions and healthcare professionals can make informed procurement decisions that align with their clinical or educational goals, ultimately improving understanding and skills related to urinary system care and treatment.