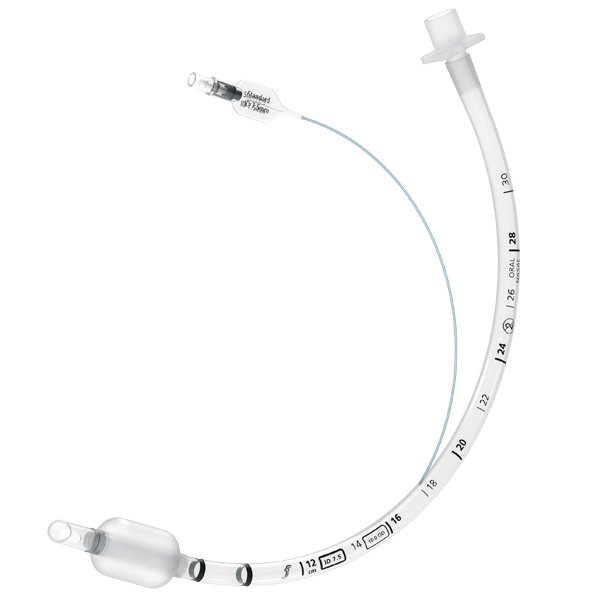
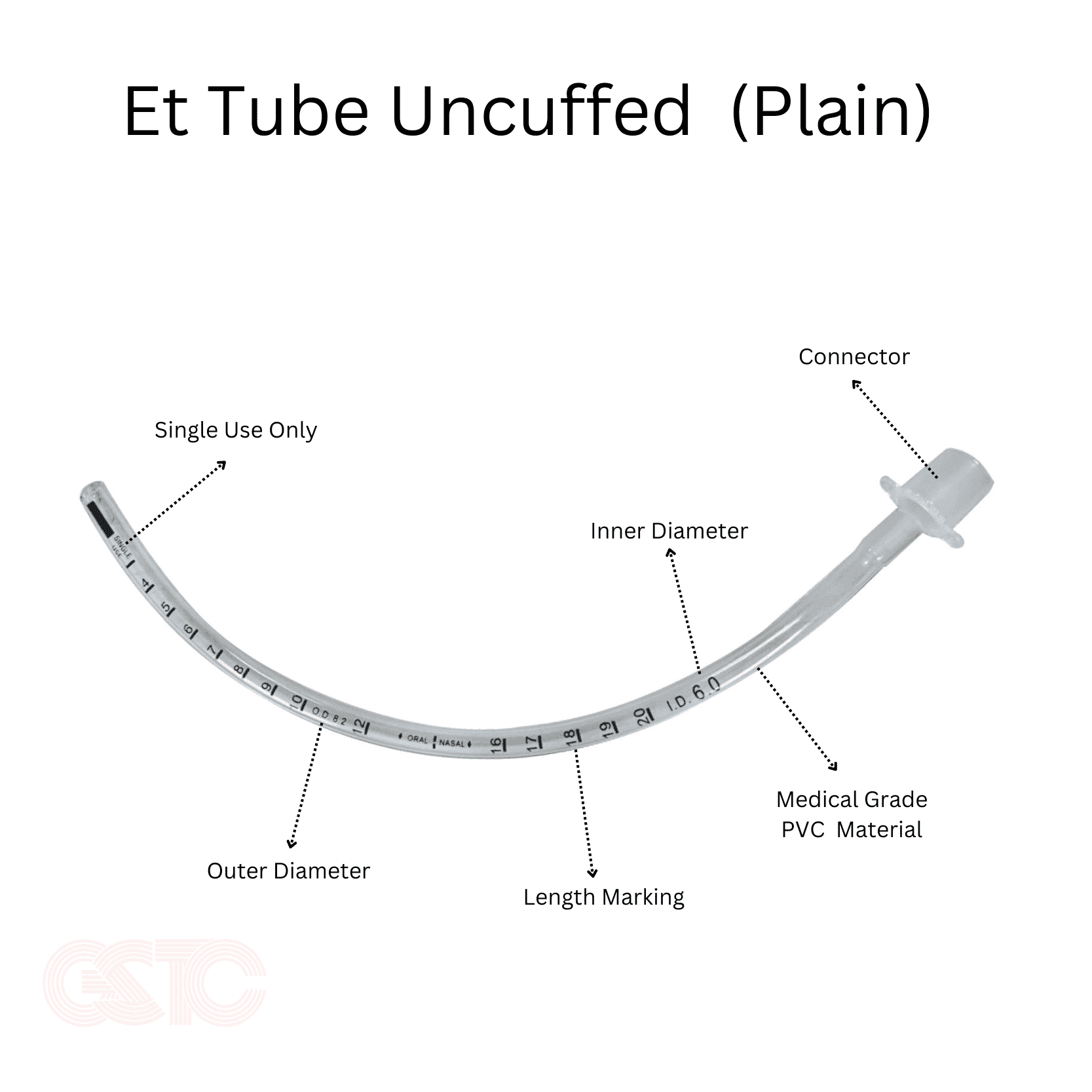
The global tracheal tube market is experiencing steady expansion, driven by rising incidences of respiratory disorders, increased ICU admissions, and growth in surgical procedures requiring anesthesia. According to Mordor Intelligence, the tracheal and endotracheal tube market was valued at approximately USD 1.2 billion in 2023 and is projected to grow at a CAGR of over 6.5% during the forecast period from 2024 to 2029. This growth is further supported by advancements in tube materials, design innovations for reduced airway trauma, and the increasing demand for specialized pediatric and neonatal tubes. Within this evolving landscape, uncuffed tracheal tubes—commonly used in pediatric and short-term ventilation applications—have gained significant traction due to their favorable safety profile and ease of use. As healthcare providers prioritize patient-specific and complication-minimizing solutions, manufacturers are focusing on enhancing biocompatibility, kink resistance, and ease of insertion. In response to these market dynamics, several key players have emerged as leaders in innovation and quality in uncuffed tracheal tube production, shaping the standard of care across acute and critical care settings.
Top 6 Uncuffed Tracheal Tube Manufacturers 2026
(Ranked by Factory Capability & Trust Score)
#1 InTube™ tracheal tube uncuffed
Domain Est. 1996
Website: intersurgical.com
Key Highlights: Intersurgical’s InTube™ uncuffed tracheal tubes are available in a variety of sizes designed for use in anaesthesia and emergency medicine….
#2 Endotracheal Tubes
Domain Est. 1996
Website: teleflex.com
Key Highlights: All endotracheal tubes are designed to feature an excellent I.D. to O.D. ratio, biocompatible materials, thin-walled low-pressure cuff design and a smooth tip ……
#3 Bivona™ Uncuffed Neonatal and Pediatric Tracheostomy Tubes
Domain Est. 1997
Website: icumed.com
Key Highlights: The Bivona Uncuffed Neonatal and Pediatric Tracheostomy Tube is designed to provide maximum patient comfort through its soft and flexible silicone construction….
#4 Cuffed, Uncuffed & VentiSeal Endotracheal Tubes
Domain Est. 1999
Website: flexicare.com
Key Highlights: Our optimal size and shape Cuffed & Uncuffed endotracheal tubes provide a secure tracheal seal, ideal for short term intubations and emergency situations….
#5 Endotracheal Tubes
Domain Est. 2000
Website: tri-anim.com
Key Highlights: 60-day returnsBlue Line® Uncuffed Nasal/Oral Endotracheal Tube features include: * Standard 15 mm ISO Connector * Uncuffed * Oral/Nasal * Siliconized PVC * Sterile….
#6 Uncuffed Endotracheal Tube
Domain Est. 2006
Website: boenmedical.com
Key Highlights: BOENMED manufactures UNCUFFED ENDOTRACHEAL TUBE. The Endotracheal tube is a flexible air duct used for inhalation anesthesia or assisted ventilation….
Expert Sourcing Insights for Uncuffed Tracheal Tube

H2: Projected 2026 Market Trends for Uncuffed Tracheal Tubes
The global market for uncuffed tracheal tubes is poised for moderate but steady growth by 2026, driven by evolving clinical practices, increasing pediatric and neonatal intensive care demands, and growing awareness of airway management safety. Uncuffed tracheal tubes, traditionally preferred in pediatric patients under eight years of age due to concerns about subglottic trauma and post-extubation complications, are experiencing shifts in usage patterns owing to advancements in tube design and emerging evidence supporting the safe use of cuffed tubes in younger populations.
One key trend shaping the 2026 outlook is the gradual adoption of high-volume, low-pressure cuffed tubes even in pediatric settings, which may limit the expansion of uncuffed tube usage in some regions. However, in low-resource and emerging healthcare environments, uncuffed tubes remain the standard of care due to cost-effectiveness, ease of use, and infrastructure limitations. This dichotomy creates a segmented market where demand for uncuffed tubes persists strongly in Asia-Pacific, Latin America, and parts of Africa.
Additionally, rising neonatal ICU admissions and expanded access to emergency airway care in ambulatory and pre-hospital settings contribute to sustained demand. Manufacturers are responding with innovations such as improved tube materials, better sealing technologies, and graduated sizing systems to enhance fit and reduce complications—efforts aimed at preserving the relevance of uncuffed designs amid competitive pressure.
Regulatory scrutiny and clinical guidelines from bodies like the American Society of Anesthesiologists (ASA) and the European Society of Paediatric and Neonatal Intensive Care (ESPNIC) continue to influence purchasing decisions, with many institutions favoring evidence-based protocols that occasionally favor cuffed tubes for better ventilation control. Nevertheless, uncuffed tubes maintain a critical niche in short-term intubations and outpatient procedures.
By 2026, the uncuffed tracheal tube market is expected to grow at a compound annual growth rate (CAGR) of approximately 4.2%, with volume sales remaining stable despite value pressures due to pricing competition. Strategic consolidation among medical device manufacturers and partnerships with public health systems in developing economies may further shape market dynamics.
In summary, while the uncuffed tracheal tube market faces challenges from technological advancements favoring cuffed alternatives, its entrenched role in specific patient populations and cost-sensitive healthcare systems ensures continued relevance through 2026.
Common Pitfalls When Sourcing Uncuffed Tracheal Tubes (Quality and Intellectual Property Concerns)
Sourcing uncuffed tracheal tubes—commonly used in pediatric and certain adult airway management scenarios—can present significant challenges, particularly in ensuring product quality and avoiding intellectual property (IP) violations. Procurement teams, healthcare providers, and distributors must be vigilant to avoid common pitfalls that can compromise patient safety, regulatory compliance, and legal integrity.
Quality-Related Pitfalls
One of the most critical concerns when sourcing uncuffed tracheal tubes is ensuring consistent product quality. Poor-quality tubes can lead to airway complications such as obstruction, leakage, or trauma during insertion.
-
Inconsistent Material Standards
Many low-cost manufacturers use substandard medical-grade PVC or alternative polymers that may lack flexibility, durability, or biocompatibility. Tubes made from inferior materials are more prone to kinking, collapsing, or causing mucosal irritation. -
Lack of Regulatory Certification
Sourcing from suppliers without valid certifications (e.g., FDA 510(k), CE marking, ISO 13485) increases the risk of non-compliant products entering the supply chain. Uncertified tubes may not meet critical performance standards for dimensional accuracy, cuff pressure (if applicable), or radiopacity. -
Poor Manufacturing Controls
Inadequate quality control processes can result in batch-to-batch variability, incorrect sizing, or defective packaging. This is especially problematic with uncuffed tubes, where precise outer diameter and bevel design are crucial for safe intubation. -
Inadequate Sterility Assurance
Many sourced tracheal tubes are supplied sterile, but poor manufacturing or packaging practices can compromise sterility. Suppliers without proper cleanroom environments or validated sterilization methods (e.g., ethylene oxide, gamma irradiation) pose infection risks.
Intellectual Property (IP) Concerns
Beyond quality, sourcing uncuffed tracheal tubes can expose buyers to intellectual property risks, particularly when dealing with third-party or copycat manufacturers.
-
Design and Patent Infringement
Leading brands often hold patents on specific tracheal tube designs, such as bevel geometry, Murphy eye placement, or connector standards. Sourcing imitations that replicate these features without licensing can lead to legal disputes, product seizures, or liability for contributory infringement. -
Trademark and Brand Confusion
Some suppliers may mimic the branding, packaging, or labeling of well-known manufacturers, leading to potential trademark violations. Even unintentional association with a protected brand can result in legal action or reputational harm. -
Lack of IP Due Diligence
Buyers may overlook the importance of verifying a supplier’s IP rights. Without proper documentation—such as freedom-to-operate (FTO) opinions or patent licenses—organizations risk importing or distributing infringing devices, especially in regulated markets like the U.S. or EU. -
Gray Market and Unauthorized Distribution
Purchasing from unauthorized distributors or gray market channels increases the likelihood of receiving counterfeit or IP-violating products. These channels often lack transparency and may sell tubes that bypass original manufacturer controls.
Conclusion
To mitigate risks, organizations should conduct thorough supplier audits, demand full regulatory documentation, and consult legal experts to assess IP compliance. Prioritizing quality and legitimacy over cost ensures patient safety and protects against legal and reputational damage.

Logistics & Compliance Guide for Uncuffed Tracheal Tubes
This guide outlines essential logistics considerations and regulatory compliance requirements for the handling, distribution, and use of Uncuffed Tracheal Tubes in medical settings. Adherence to these guidelines ensures patient safety, regulatory conformity, and efficient supply chain operations.
Regulatory Classification and Approval
Uncuffed Tracheal Tubes are classified as medical devices, typically falling under Class II (or equivalent, such as Class Ir in the EU under the MDR) due to their invasive nature and direct contact with the respiratory system. They require regulatory clearance or approval from competent authorities such as the U.S. FDA (510(k) clearance), Health Canada (Medical Device License), or conformity assessment under the EU Medical Device Regulation (MDR) for CE marking. Manufacturers must maintain valid Technical Files, Quality Management System (QMS) certification (e.g., ISO 13485), and comply with post-market surveillance requirements.
Labeling and Packaging Requirements
All packaging must comply with applicable regulations (e.g., FDA 21 CFR Part 801, EU MDR Annex I), including clear labeling with the device name (“Uncuffed Tracheal Tube”), size (ID in mm), length, lot number, expiration date, manufacturer details, and single-use symbol. Sterility indicators must be present if the device is supplied sterile. Labels must be durable, legible, and include necessary warnings (e.g., “For single patient use only,” “Sterile unless package is damaged”). Language requirements vary by market (e.g., bilingual labeling in Canada, multilingual in EU member states).
Storage and Environmental Conditions
Uncuffed Tracheal Tubes should be stored in a clean, dry, temperature-controlled environment, typically between 15°C and 30°C (59°F to 86°F), and protected from direct sunlight and excessive humidity. Storage areas must prevent contamination and physical damage. Devices should remain in original sealed packaging until point of use. Inventory rotation (FIFO – First In, First Out) must be strictly enforced to prevent the use of expired products.
Transportation and Distribution
During transportation, devices must be protected from extreme temperatures, moisture, and physical shock. Use validated shipping containers and monitoring (e.g., temperature data loggers if required) to ensure product integrity. Distributors and logistics partners must be authorized and comply with Good Distribution Practice (GDP) for medical devices. Temperature excursions during transit must be documented and assessed for impact on sterility and material integrity.
Inventory Management and Traceability
Robust inventory management systems are required to track lot numbers, expiration dates, and quantities. Implement systems that support full traceability from manufacturer to end-user, in compliance with UDI (Unique Device Identification) requirements (e.g., FDA UDI Rule, EU MDR). Each device or packaging level must bear a UDI barcode, enabling accurate tracking in electronic health records and adverse event reporting.
Single-Use and Reuse Policy
Uncuffed Tracheal Tubes are strictly for single-patient, single-use only. Reuse is prohibited and violates regulatory standards and infection control guidelines. Institutions must have clear policies and staff training to prevent reuse. Proper disposal as medical waste must follow local regulations (e.g., biohazard protocols).
Adverse Event Reporting and Post-Market Surveillance
Healthcare providers and distributors are required to report any adverse events, device malfunctions, or suspected product defects to the manufacturer and relevant regulatory authority (e.g., FDA MedWatch, EUDAMED). Manufacturers must maintain a post-market surveillance plan to collect and analyze feedback, conduct trend analysis, and initiate field safety corrective actions if necessary.
Training and Clinical Use Compliance
Only trained healthcare professionals should insert and manage uncuffed tracheal tubes. Institutions must ensure staff competency through regular training on proper selection (based on patient age, weight, and anatomy), insertion techniques, securement, and monitoring. Use must align with clinical guidelines and manufacturer’s Instructions for Use (IFU), which must be accessible at the point of care.
Import/Export Documentation
International shipment requires accurate customs documentation, including certificates of free sale, certificates of origin, and proof of regulatory clearance in the destination country. Exporters must comply with import regulations of the receiving country and ensure that devices meet local language and labeling requirements. Restricted substance compliance (e.g., RoHS, REACH) must also be verified where applicable.
Conclusion for Sourcing Uncuffed Tracheal Tubes:
Sourcing uncuffed tracheal tubes requires careful consideration of clinical guidelines, patient demographics—particularly age and weight—and regulatory compliance. These devices are primarily indicated for pediatric patients, especially those under 8 years of age, where the risk of subglottic injury from cuff pressure is higher. When sourcing, it is essential to prioritize tubes that meet international standards (e.g., ISO 5361), ensure biocompatibility, and offer appropriate sizing accuracy. Engaging with reputable suppliers and manufacturers with proven quality management systems is critical to ensuring device safety and performance. Additionally, healthcare providers should consider availability, cost-effectiveness, and logistical factors such as supply chain reliability to maintain continuity of care. Ultimately, the goal is to procure uncuffed tracheal tubes that support safe and effective airway management while minimizing complications in vulnerable patient populations.