The global strength training equipment market, driven by rising fitness awareness and increased investment in commercial and home gyms, is projected to grow at a CAGR of 5.8% from 2023 to 2030, according to Grand View Research. As demand for targeted lower-body training solutions intensifies, the tibialis machine—a specialized tool for strengthening the anterior muscles of the lower leg—has gained traction among athletes, rehab centers, and functional fitness facilities. This growing niche is supported by advancements in biomechanics and training science, fueling innovation among manufacturers. Based on market presence, product quality, and technological integration, we’ve identified the top 6 tibialis machine manufacturers leading this segment. These companies are not only meeting current demand but are shaping the future of lower-leg strength and injury prevention training.
Top 6 Tibialis Machine Manufacturers 2026
(Ranked by Factory Capability & Trust Score)
#1 Plate Loaded Tibia Dorsi
Domain Est. 1995
Website: lifefitness.com
Key Highlights: Our Hammer Strength Tibia Dorsi-Flexion machine zeroes in on this vital muscle, strengthening the muscles responsible for ankle dorsiflexion….
#2 Commercial Dorsiflexion Machines
Domain Est. 2011
Website: atlantisstrength.com
Key Highlights: Enhance leg workouts with a commercial dorsiflexion machine that targets the tibialis while reducing spinal load. Ideal for gyms focused on lower-leg strength….
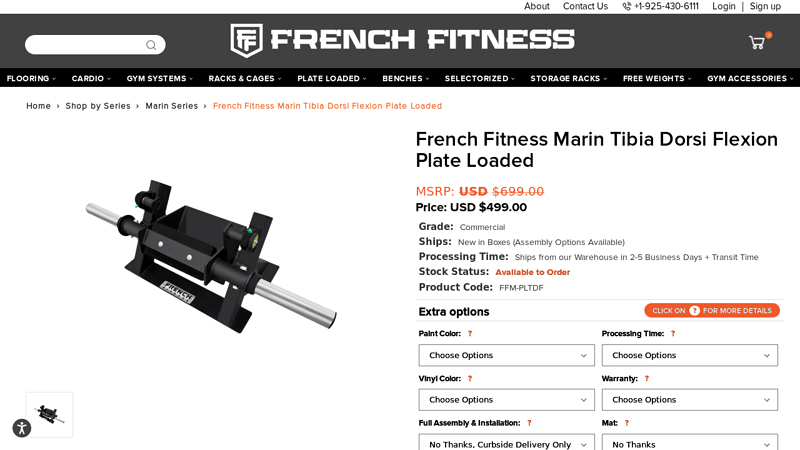
#3 French Fitness Marin Tibia Dorsi Flexion Plate Loaded
Domain Est. 2016
Website: frenchfitness.com
Key Highlights: In stock 14-day returnsThis machine allows users to perform either one-leg standing or two-feet seated tibia raises, accommodating different strength levels and training preference…
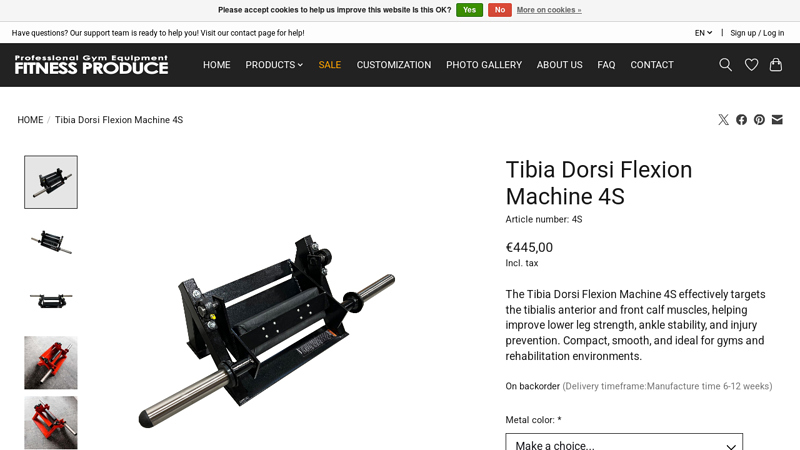
#4 Tibia Dorsi Flexion Machine 4S
Domain Est. 2018
Website: fitnessproduce.nl
Key Highlights: Train the tibialis anterior with the 4S Tibia Dorsi Flexion Machine. Compact, smooth, and perfect for strengthening lower leg muscles and ankle stability….
#5 Tib Bars & Tibialis Training Equipment
Domain Est. 2021
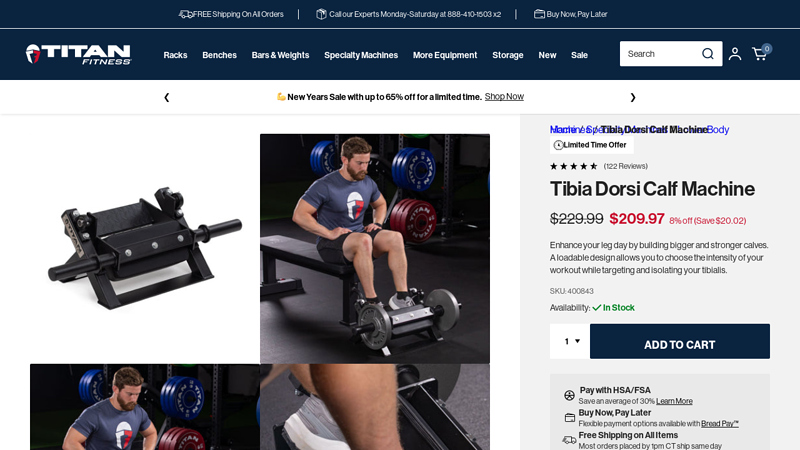
#6 Tibia Dorsi Calf Machine
Expert Sourcing Insights for Tibialis Machine

2026 Market Trends for Tibialis Machines
The market for tibialis machines—specialized rehabilitation and strength training devices targeting the tibialis anterior and posterior muscles—will experience notable evolution by 2026, shaped by advancements in technology, shifting healthcare demands, and growing awareness of musculoskeletal health. Key trends include:
Rising Demand Driven by Sports Medicine and Injury Prevention
The increasing participation in high-impact sports and fitness activities is fueling demand for targeted lower-leg rehabilitation tools. Tibialis machines are gaining prominence in athletic training facilities and physical therapy clinics due to their efficacy in preventing and rehabilitating conditions like shin splints, tendonitis, and ankle instability. By 2026, a greater emphasis on injury prevention programs in both amateur and professional sports will drive adoption of these machines, particularly in preventive care models.
Integration of Smart Technology and Data Analytics
By 2026, tibialis machines are expected to incorporate advanced sensors, real-time feedback systems, and connectivity features (e.g., Bluetooth, mobile app integration). These enhancements will allow users and clinicians to track progress, customize resistance, and monitor muscle activation through biofeedback. AI-driven analytics could recommend personalized training protocols, improving rehabilitation outcomes and user engagement. This trend aligns with the broader digital transformation in fitness and medical rehabilitation equipment.
Expansion in Home-Based Rehabilitation Solutions
The post-pandemic shift toward home healthcare and tele-rehabilitation continues to influence the market. By 2026, compact, user-friendly tibialis machines designed for home use will become more prevalent. These devices will cater to aging populations and individuals managing chronic lower-limb conditions, supported by remote monitoring capabilities that allow therapists to supervise recovery remotely.
Focus on Personalization and Accessibility
Manufacturers will increasingly prioritize ergonomic design, adjustable resistance levels, and inclusion for users with varying mobility levels. Customizable programs based on individual biomechanics will enhance therapeutic effectiveness. Additionally, efforts to reduce cost barriers through modular designs and insurance reimbursement pathways will improve market accessibility.
Growth in Emerging Markets and Preventive Health Initiatives
Expanding healthcare infrastructure in regions like Asia-Pacific and Latin America, combined with rising awareness of musculoskeletal health, will contribute to market growth. Public and private health initiatives promoting preventive care are likely to include tibialis strengthening as part of fall-prevention programs for older adults, further boosting demand.
In summary, the tibialis machine market in 2026 will be characterized by technological innovation, integration into personalized and preventive care models, and a shift toward decentralized, data-driven rehabilitation—positioning these devices as essential tools in both clinical and home settings.

Common Pitfalls When Sourcing a Tibialis Machine (Quality, IP)
Sourcing a tibialis machine—used in biomechanics, rehabilitation, or sports science research—requires careful attention to both quality and intellectual property (IP) concerns. Overlooking these aspects can lead to subpar performance, legal risks, and financial losses. Below are key pitfalls to avoid:
Quality-Related Pitfalls
1. Inadequate Mechanical Precision and Calibration
A poorly manufactured tibialis machine may lack the precision needed for accurate data collection. Issues such as inconsistent resistance, misaligned components, or poor sensor calibration can compromise research validity or therapeutic outcomes. Always verify tolerances, material quality, and calibration protocols with the supplier.
2. Use of Substandard Materials and Components
Lower-cost machines may use inferior metals, plastics, or electronic sensors that degrade quickly under repeated use. This leads to frequent maintenance, downtime, and unreliable results. Insist on detailed specifications for materials and request third-party test reports when available.
3. Lack of Regulatory Compliance or Safety Certifications
Machines not compliant with medical device standards (e.g., ISO 13485, IEC 60601) or lacking CE/FDA markings may pose safety risks and hinder deployment in clinical or research settings. Confirm that the product meets relevant safety and performance regulations for your region.
4. Insufficient Documentation and Technical Support
Poor user manuals, absence of maintenance guides, or unresponsive technical support can severely impact usability. Ensure the supplier provides comprehensive documentation, training, and ongoing support before finalizing procurement.
Intellectual Property-Related Pitfalls
1. Risk of Infringing on Patented Designs
Many tibialis machines incorporate patented mechanisms (e.g., resistance systems, sensor integration, motion control). Sourcing from a manufacturer that copies protected technology exposes your organization to legal liability. Conduct due diligence on the machine’s design origins and request IP indemnification from the supplier.
2. Unclear Ownership of Custom Modifications
If you request custom features or software integration, failing to define IP ownership in the contract may result in the manufacturer retaining rights to your innovations. Clearly negotiate and document IP rights for any co-developed or modified components.
3. Use of Proprietary Software Without Licensing
Some machines rely on proprietary control software. Unauthorized use, duplication, or reverse-engineering of this software can violate copyright or licensing agreements. Ensure you receive proper software licenses and understand usage rights.
4. Counterfeit or Clone Products
Be cautious of suppliers offering high-performance machines at unusually low prices—these may be unlicensed clones infringing on original designs. Verify the manufacturer’s authenticity, request proof of IP ownership, and avoid gray-market distributors.
Best Practices to Mitigate Risks
- Conduct supplier audits and request references from existing clients.
- Engage legal counsel to review contracts, especially clauses on liability, warranties, and IP.
- Prototype testing before bulk ordering to evaluate quality and performance.
- Verify compliance certifications and request up-to-date documentation.
By proactively addressing these quality and IP pitfalls, organizations can ensure reliable, legally compliant tibialis machine procurement that supports accurate research and safe application.

Logistics & Compliance Guide for Tibialis Machine
Product Overview
The Tibialis Machine is a medical or rehabilitation device designed to assess and strengthen the tibialis anterior and posterior muscles in the lower leg. Proper logistics handling and regulatory compliance are essential for safe distribution, installation, and use.
Regulatory Classification
The Tibialis Machine is typically classified as a Class I or Class II medical device under regulatory frameworks such as the U.S. FDA 21 CFR Part 890 (Physical Medicine Devices) or EU MDR (Regulation (EU) 2017/745), depending on its intended use and risk profile. Confirm classification with local regulatory authorities prior to distribution.
Regulatory Compliance Requirements
United States (FDA)
- Register the device with the FDA and obtain a Device Listing Number.
- Ensure compliance with Quality System Regulation (QSR) 21 CFR Part 820.
- Maintain Technical File and Design History File (DHF).
- Labeling must include: device name, manufacturer details, UDI (Unique Device Identifier), and intended use.
European Union (EU MDR)
- Obtain CE marking via conformity assessment (typically self-certification for Class I, Notified Body involvement for Class IIa/IIb).
- Compile a comprehensive Technical Documentation file per Annex II and III of EU MDR.
- Appoint an Authorized Representative (EC Rep) if the manufacturer is based outside the EU.
- Register devices in EUDAMED upon availability.
Other Markets (Canada, Australia, etc.)
- Canada: License under Health Canada’s Medical Devices Regulations (SOR/98-282); obtain a Medical Device License (MDL).
- Australia: Include in the Australian Register of Therapeutic Goods (ARTG) via the TGA.
- Consult local regulatory bodies for country-specific requirements.
Labeling & Packaging Standards
- Include UDI barcode compliant with FDA and EU standards.
- Use tamper-evident packaging for sterile or sensitive components.
- Label with storage conditions (e.g., temperature range: 10–30°C), handling symbols (e.g., “Fragile,” “This Side Up”).
- Multilingual labeling required for international shipments (English plus local language(s)).
Shipping & Transportation
- Use certified freight forwarders experienced in medical device logistics.
- Maintain temperature-controlled transport if required by device specifications.
- Secure machines with shock-absorbing materials to prevent damage during transit.
- Provide detailed shipping documentation: commercial invoice, packing list, certificate of origin, and regulatory certificates (e.g., CE, FDA registration).
Import & Customs Clearance
- Ensure Harmonized System (HS) code classification (e.g., 9019.10 for therapeutic appliances).
- Submit required import documentation, including regulatory permits (e.g., Import License, Certificate of Free Sale).
- Partner with a customs broker familiar with medical device regulations in destination countries.
Installation & Commissioning
- Provide trained technicians to install and calibrate the device per manufacturer specifications.
- Document installation and provide user training, including safety and operational procedures.
- Deliver user manuals, service guides, and warranty information in the local language.
Post-Market Surveillance & Vigilance
- Establish a system for reporting adverse events and field safety corrective actions (FSCAs).
- Comply with FDA MedWatch (Form 3500A) and EU EUDAMED reporting requirements.
- Maintain a Quality Management System (QMS) for continuous improvement and audit readiness.
Maintenance & Servicing
- Schedule regular preventive maintenance as per manufacturer guidelines.
- Use only authorized service personnel and genuine spare parts.
- Keep service logs and update device software/firmware as released.
Disposal & End-of-Life
- Follow local regulations for disposal of medical devices (e.g., EU WEEE Directive, local biomedical waste rules).
- Offer take-back or recycling programs where applicable.
- Deactivate and securely erase any stored patient data prior to disposal.
Contact Information
For compliance inquiries or support:
Regulatory Affairs: [email protected]
Technical Support: [email protected]
Global Logistics: [email protected]
Conclusion for Sourcing a Tibialis Machine:
After thorough evaluation of available options, budget considerations, supplier reliability, and equipment specifications, sourcing a tibialis machine is a strategic investment in enhancing rehabilitation and strength training capabilities—particularly for athletes, post-surgical patients, and individuals with lower limb instability. The selected machine should offer targeted resistance for the tibialis anterior muscle, adjustable settings for progressive loading, and ergonomic design to ensure user safety and comfort.
Sourcing from reputable manufacturers with certifications (such as ISO and FDA compliance), strong customer support, and service agreements will ensure long-term durability and performance. Additionally, considering user feedback and conducting product demonstrations prior to purchase can further validate the suitability of the machine for clinical or training environments.
In conclusion, procuring a high-quality tibialis machine not only supports specialized neuromuscular rehabilitation and athletic performance but also demonstrates a commitment to evidence-based, functional strength training. With proper integration into therapeutic or fitness programs, this equipment can significantly improve dorsiflexion strength, reduce injury risk, and enhance overall lower limb function.