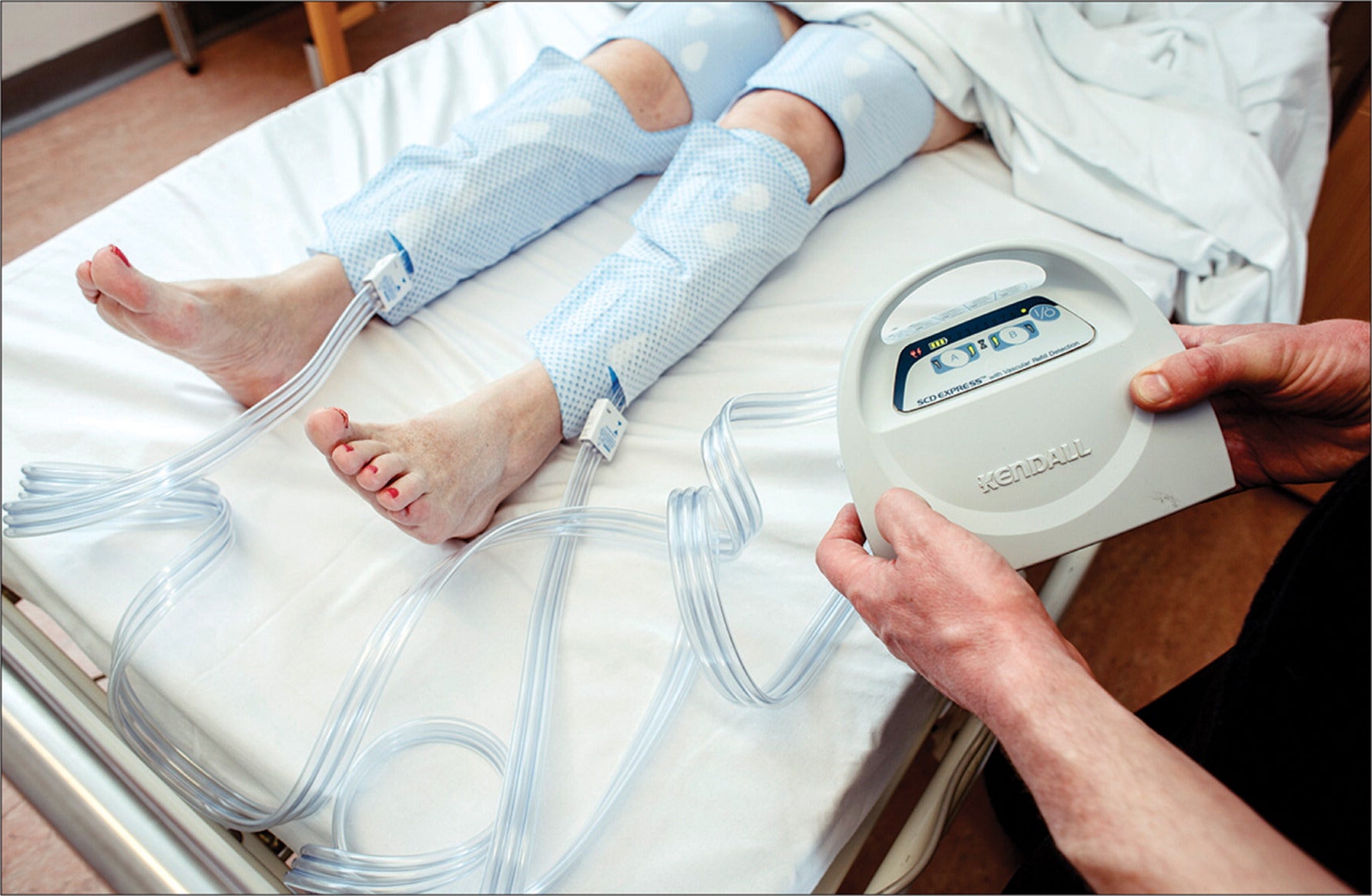
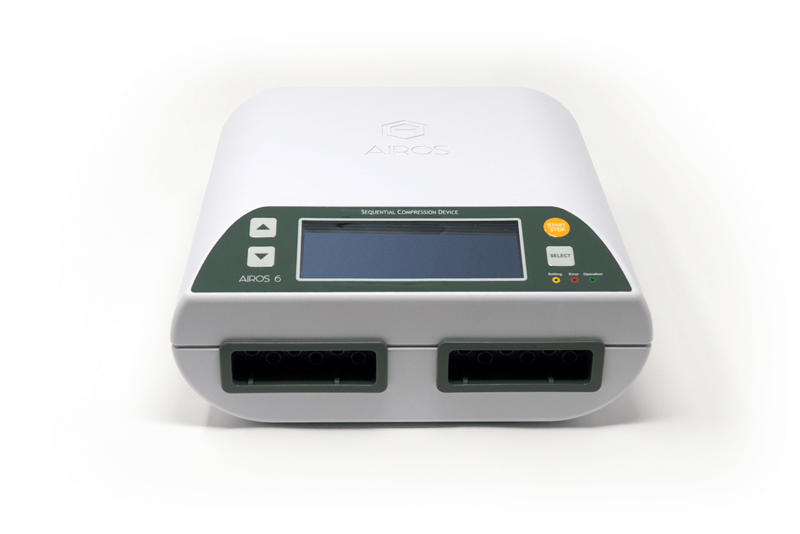
The global sequential compression device market is experiencing robust growth, driven by the rising prevalence of venous disorders, increased post-surgical care demands, and a growing preference for home-based therapy solutions. According to Grand View Research, the global compression therapy market size was valued at USD 2.1 billion in 2022 and is expected to expand at a compound annual growth rate (CAGR) of 7.8% from 2023 to 2030. This expansion is fueled by an aging population, escalating rates of deep vein thrombosis (DVT), and heightened awareness of non-invasive treatments. Mordor Intelligence further projects that increasing hospital discharges and the shift toward patient-centric care models are accelerating adoption of home-use medical devices, including sequential compression devices (SCDs). As healthcare systems emphasize reduced hospital stays and lower readmission rates, the demand for reliable, easy-to-use, and clinically effective SCDs in home settings has surged. This evolving landscape has prompted a competitive manufacturing environment, with innovation focusing on portability, user comfort, and smart integration. The following list highlights the top 10 manufacturers leading this segment with proven product performance, regulatory compliance, and strong clinical support.
Top 10 Sequential Compression Device For Home Use Manufacturers 2026
(Ranked by Factory Capability & Trust Score)
#1 AIROS Medical
Domain Est. 2016
Website: airosmedical.com
Key Highlights: Our compression therapy device technology helps thousands of people battling lymphatic and venous disorders live better….
#2 SCD Machines (Sequential Compression Devices)
Domain Est. 1996
#3 Intermittent Pneumatic Compression (IPC) Devices
Domain Est. 1996
Website: cardinalhealth.com
Key Highlights: The PlasmaFlow™ Portable Compression Device provides continuous, hospital-quality DVT prevention to patients recovering at home. Small white 10 by 10 pixel box ……
#4 Dynamic (Pneumatic) Compression Therapy
Domain Est. 1998
Website: woundsource.com
Key Highlights: The IC-1200-WH Vascu-Ease Portable DVT System is intended to be an easy-to-use sequential compression system, for use in the hospital or home setting to help ……
#5 ReMarx Medical Services
Domain Est. 2002
Website: remarxservices.com
Key Highlights: The AIROS 6 Sequential Compression Device is a 6-chamber device that treats lymphedema and chronic venous disorders using gradient sequential compression….
#6 SCDS DVT Prevention
Domain Est. 2009
Website: compressionsolutions.us
Key Highlights: The sleeves connect directly to the pump, providing alternating, intermittent sequential compression to help prevent deep vein thrombosis….
#7 Precision Medical Products:
Domain Est. 2013
Website: pmpmed.com
Key Highlights: Precision Medical has designed the leading mobile mechanical blood clot prevention solution. Our innovative approach to post-surgery recovery and prevention…
#8 LX7 Compression Device
Domain Est. 2016
Website: woundreference.com
Key Highlights: LX7 Home Compression Device is a sequential gradient compression device. INTENDED USES: Promotes blood and lymph circulation in conditions such as lymphedema, ……
#9 Sequential Compression Device
Domain Est. 2016
Website: compressionmedical.com
Key Highlights: Free deliveryWe have great sequential compression devices and lyphedema pumps at your disposal! Check our website out for more information….
#10 Vekroosan
Website: vekroosan.com.au
Key Highlights: Vekroosan is an easy-to-use, mobile calf compression device designed to prevent and treat Deep Vein Thrombosis, Chronic Venous Disorder and related vein ……
Expert Sourcing Insights for Sequential Compression Device For Home Use

H2: 2026 Market Trends for Sequential Compression Devices for Home Use
The global market for Sequential Compression Devices (SCDs) for home use is poised for significant growth and transformation by 2026, driven by demographic shifts, technological innovation, and evolving healthcare delivery models. As chronic conditions like lymphedema, deep vein thrombosis (DVT), and post-surgical recovery become increasingly prevalent, the demand for effective, non-invasive home-based therapies continues to rise.
One of the primary drivers shaping the 2026 market is the growing aging population worldwide. Older adults are more susceptible to circulatory disorders and mobility limitations, increasing their reliance on home medical devices. This demographic trend is prompting both patients and healthcare providers to favor at-home solutions that improve quality of life while reducing hospital readmissions and outpatient visits.
Advancements in device technology are also reshaping the SCD market. By 2026, manufacturers are expected to focus on developing lightweight, portable, and user-friendly devices with enhanced programmability and data connectivity. Integration with mobile health (mHealth) applications and remote patient monitoring systems allows for real-time tracking of therapy adherence and clinical outcomes, facilitating better care coordination between patients and providers.
Another key trend is the shift toward value-based care and cost containment in healthcare systems. Payers and providers are increasingly incentivizing home-based treatments to reduce the burden on acute care facilities. Reimbursement policies in several regions, including the U.S. and parts of Europe, are gradually expanding coverage for home-use SCDs, thereby improving patient access and market penetration.
Additionally, heightened patient awareness and the normalization of self-care technologies are contributing to rising consumer demand. Educational campaigns and telehealth consultations are empowering patients to manage chronic conditions proactively, further fueling adoption of SCDs in non-clinical settings.
Competitive dynamics are intensifying, with both established medical device companies and emerging startups investing in R&D to differentiate their products through comfort, ease of use, and smart features. Strategic partnerships with home healthcare providers and digital health platforms are expected to become more common as companies aim to offer integrated care solutions.
In summary, the 2026 market for Sequential Compression Devices for home use will be characterized by innovation, increased accessibility, and integration into broader digital health ecosystems. These trends collectively signal a robust expansion of the market, with a strong emphasis on patient-centric, cost-effective, and data-driven care delivery models.

Common Pitfalls When Sourcing Sequential Compression Devices for Home Use (Quality, IP)
Sourcing a Sequential Compression Device (SCD) for home use requires careful consideration, especially regarding product quality and intellectual property (IP) concerns. Patients and caregivers often face several challenges when selecting a reliable and legally compliant device. Below are some common pitfalls to avoid.
Choosing Low-Quality or Non-Certified Devices
One of the most significant risks is purchasing substandard devices that lack proper medical certifications. Many low-cost SCDs available online may not meet FDA, CE, or other regulatory standards. These devices can be ineffective or even dangerous, leading to inadequate therapy or skin injuries. Always verify that the device is cleared by relevant regulatory bodies and designed specifically for medical use.
Ignoring Intellectual Property Infringement Risks
Some manufacturers produce devices that closely mimic patented technologies from leading medical companies. These “copycat” products may infringe on intellectual property rights, potentially leading to legal issues for distributors or even end-users in rare cases. Using or distributing counterfeit devices not only poses legal risks but also indicates poor quality control and lack of innovation.
Purchasing from Unverified Online Sellers
The rise of e-commerce has made it easier to access medical devices, but it has also increased the risk of encountering counterfeit or unauthorized resellers. These sellers may offer devices at reduced prices but often lack technical support, warranties, or authenticity guarantees. Always purchase from authorized dealers or directly from reputable manufacturers.
Overlooking Device Compatibility and Support
Low-quality or IP-infringing devices often lack proper technical documentation, customer support, or compatible replacement parts (such as sleeves or hoses). This can lead to long-term usability issues, especially for home users who rely on consistent therapy. Ensure the supplier provides ongoing support and access to consumables.
Assuming All Devices Offer the Same Therapy
Not all sequential compression devices deliver the same level of pneumatic compression or follow clinically validated pressure sequences. Imitation devices may not replicate the therapeutic profiles of original, patented models. This can reduce clinical effectiveness and compromise patient outcomes.
Failure to Verify Warranty and Service Options
Many counterfeit or low-quality devices come with limited or no warranty. In contrast, legitimate manufacturers typically offer service programs, repair options, and technical assistance. Always check warranty terms and service availability before making a purchase.
By being aware of these pitfalls—especially those related to quality assurance and intellectual property—patients and caregivers can make safer, more informed decisions when sourcing Sequential Compression Devices for home use.
Logistics & Compliance Guide for Sequential Compression Devices for Home Use
Overview and Purpose
This guide outlines the essential logistics and compliance considerations for the distribution, use, and management of Sequential Compression Devices (SCDs) intended for home care settings. SCDs are medical devices used to prevent deep vein thrombosis (DVT) by improving blood circulation in the legs through intermittent pneumatic compression. Ensuring proper logistics and regulatory compliance is critical for patient safety, reimbursement, and legal adherence.
Regulatory Classification and Approval
Sequential Compression Devices are classified as Class II medical devices by the U.S. Food and Drug Administration (FDA) under 21 CFR 890.5925. They require 510(k) premarket notification clearance to demonstrate substantial equivalence to a legally marketed predicate device. Manufacturers must ensure their devices are listed with the FDA and produced under a Quality Management System compliant with FDA’s Quality System Regulation (QSR), 21 CFR Part 820.
Outside the U.S., compliance with regional regulations is required:
– European Union: Devices must meet requirements under the Medical Device Regulation (MDR) (EU) 2017/745 and carry the CE marking.
– Canada: Devices require a Medical Device License from Health Canada under the Medical Devices Regulations (SOR/98-282).
– Other Countries: Local regulatory approvals (e.g., TGA in Australia, PMDA in Japan) may apply and must be obtained prior to distribution.
Prescription and Medical Necessity Requirements
SCDs for home use are typically prescribed by a licensed healthcare provider (e.g., physician, nurse practitioner). A written prescription must include:
– Patient diagnosis (e.g., post-surgical immobility, high risk for DVT)
– Device specifications (e.g., model number, limb coverage)
– Duration of use
– Frequency and settings (if applicable)
Documentation of medical necessity is required for insurance reimbursement, particularly for Medicare, Medicaid, and private insurers. Supporting clinical documentation (e.g., physician notes, risk assessment) must be maintained.
Reimbursement and Coding
Home-use SCDs are often covered under Medicare Part B Durable Medical Equipment (DME) benefits when medically necessary. Key billing codes include:
– HCPCS Code E0043: Pneumatic compression pump, any type, for home use
– HCPCS Code A6547: Replacement inflatable garment (each)
Billing entities must be accredited DME suppliers enrolled in Medicare and comply with the DMEPOS Supplier Standards. Failure to meet documentation or accreditation requirements may result in claim denials or audits.
Distribution and Supply Chain Logistics
Distribution of SCDs involves coordination among manufacturers, DME suppliers, and home health agencies. Key logistics steps include:
– Order Fulfillment: Process prescriptions and verify insurance eligibility.
– Device Shipping: Use reliable carriers with tracking; ensure devices are securely packaged and include user manuals, accessories, and safety information.
– Inventory Management: Maintain adequate stock levels, track serial numbers, and manage recalls or updates.
– Patient Delivery & Setup: Arrange for timely delivery and provide initial device setup and patient/caregiver training.
Patient Training and Education
Effective use of SCDs requires comprehensive patient and caregiver education, including:
– Assembly and operation of the device
– Proper limb sleeve application
– Daily usage schedule and duration
– Cleaning and maintenance of sleeves and pump
– Recognizing signs of skin irritation or device malfunction
– Emergency shutdown procedures
Training must be documented and provided in the patient’s preferred language and literacy level.
Maintenance, Repairs, and Recalls
Suppliers are responsible for:
– Providing replacement parts (e.g., sleeves, hoses) as needed
– Offering technical support and troubleshooting
– Responding promptly to device malfunctions
– Implementing FDA-mandated recalls or safety alerts
A system for tracking device performance and adverse events must be maintained and reported to the FDA via the MedWatch program when required.
Data Privacy and HIPAA Compliance
When handling patient information (e.g., prescriptions, delivery addresses, usage data), organizations must comply with the Health Insurance Portability and Accountability Act (HIPAA). This includes:
– Securing electronic protected health information (ePHI)
– Using Business Associate Agreements (BAAs) with third-party vendors
– Training staff on privacy policies
– Limiting data access to authorized personnel only
Documentation and Record Retention
Maintain comprehensive records for a minimum of seven years (or as required by state/federal law), including:
– Prescriptions and physician orders
– Proof of delivery and patient acknowledgment
– Training logs
– Billing and reimbursement records
– Device serial numbers and maintenance history
These records support audits, claims defense, and regulatory inspections.
Conclusion
Successful logistics and compliance for home-use Sequential Compression Devices require adherence to medical, regulatory, and operational standards. Stakeholders—including manufacturers, suppliers, clinicians, and patients—must collaborate to ensure safe, effective, and legally compliant use of these essential medical devices. Regular review of regulatory updates and internal compliance protocols is recommended to maintain ongoing adherence.
Conclusion: Sourcing a Sequential Compression Device for Home Use
In conclusion, sourcing a sequential compression device (SCD) for home use is a valuable step in supporting effective circulation, reducing the risk of deep vein thrombosis (DVT), and enhancing recovery—particularly for individuals with limited mobility, post-surgical patients, or those managing chronic circulatory conditions. When selecting a device, key considerations include ease of use, portability, comfort, adjustable settings, safety certifications, and compatibility with prescribed therapy protocols.
After evaluating various models, brands, and supplier options, it is evident that investing in a reliable, FDA-cleared, and clinically supported SCD system ensures both therapeutic efficacy and patient compliance. Additionally, choosing a device with responsive customer support, accessible replacement parts, and insurance or reimbursement compatibility can significantly improve the long-term usability and affordability.
Ultimately, consultation with a healthcare provider is essential to determine the appropriate device specifications and usage guidelines. With the right sequential compression device, patients can safely and effectively manage their circulatory health in the comfort and convenience of their homes, promoting better outcomes and improved quality of life.