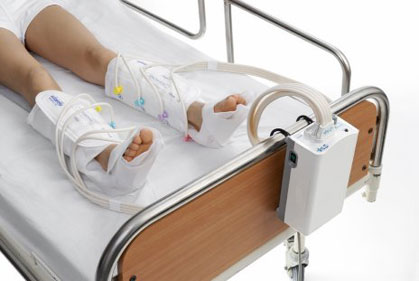
The global sequential compression device (SCD) market is experiencing robust growth, driven by rising prevalence of deep vein thrombosis (DVT), increasing surgical procedures, and growing emphasis on non-invasive thromboprophylaxis. According to a report by Mordor Intelligence, the global compression therapy devices market was valued at USD 2.3 billion in 2023 and is projected to reach USD 3.4 billion by 2029, growing at a CAGR of approximately 6.8% during the forecast period. Similarly, Grand View Research estimates the market to expand at a CAGR of over 7% from 2024 to 2030, citing advancements in portable and programmable devices as key growth catalysts. As healthcare providers prioritize patient mobility and home-based care, demand for reliable, efficient SCD systems continues to surge. In this evolving landscape, seven manufacturers have emerged as leaders—combining innovation, clinical efficacy, and scalability to shape the future of preventive vascular care.
Top 7 Scd Sequential Compression Device Manufacturers 2026
(Ranked by Factory Capability & Trust Score)
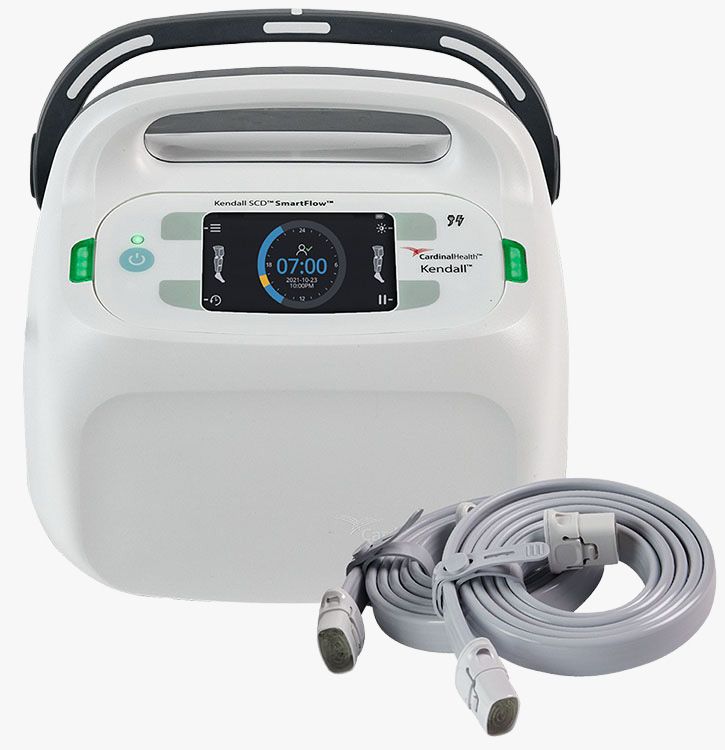
#1 Kendall SCD SmartFlow™ Controller
Domain Est. 1996
Website: cardinalhealth.com
Key Highlights: The Kendall SCD SmartFlow™ Controller applies intermittent pneumatic compression to help increase venous blood flow. How to buy. Reach out to our team for ……
#2 SCD Machines (Sequential Compression Devices)
Domain Est. 1996
#3 DVT garments and SCD sleeves
Domain Est. 1997
Website: arjo.com
Key Highlights: SCD sleeces and VTE prevention garments from Arjo. Uniform and sequential compression garments for patient comfort and high-quality care….
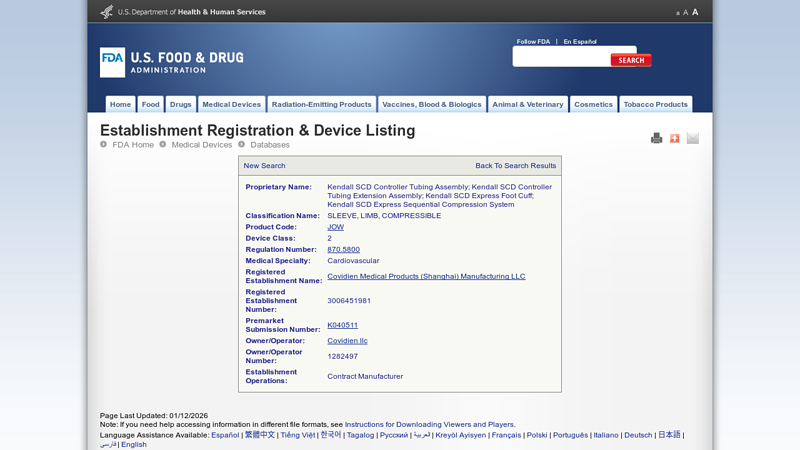
#4 Establishment Registration & Device Listing
Domain Est. 2000
Website: accessdata.fda.gov
Key Highlights: … SCD Express Foot Cuff; Kendall SCD Express Sequential Compression System. Classification Name: SLEEVE, LIMB, COMPRESSIBLE. Product Code: JOW6. Device Class: 2….
#5 Sequential Compression Devices for Treatment of Restless Legs …
Domain Est. 2000
Website: clinicaltrials.gov
Key Highlights: The purpose of this study is to determine if sequential compression devices (SCD) when worn for an hour per day by patients suffering from Restless Legs ……
#6 SCDS DVT Prevention
Domain Est. 2009
Website: compressionsolutions.us
Key Highlights: Sequential Compression Devices (SCDs) are used to prevent deep vein thrombosis after surgery for patients at risk. Without proper preventative care, DVT can be ……
#7 Precision Medical Products:
Domain Est. 2013
Website: pmpmed.com
Key Highlights: Precision Medical has designed the leading mobile mechanical blood clot prevention solution. Our innovative approach to post-surgery recovery and prevention…
Expert Sourcing Insights for Scd Sequential Compression Device

H2: 2026 Market Trends for SCD (Sequential Compression Device)
The global market for Sequential Compression Devices (SCDs), particularly in the context of venous thromboembolism (VTE) prevention, is poised for significant evolution by 2026. Driven by demographic shifts, technological innovation, and healthcare policy developments, the SCD market is expected to expand steadily with notable regional and segment-specific dynamics.
-
Growing Prevalence of VTE and Chronic Conditions
By 2026, the increasing global burden of cardiovascular diseases, obesity, and aging populations will continue to fuel demand for SCDs. An estimated rise in post-surgical and immobilized patients—especially in orthopedic and critical care settings—will reinforce the need for effective mechanical prophylaxis. The World Health Organization projects that by 2030, over 70% of deaths globally will be attributable to chronic diseases, many of which elevate VTE risk, thereby increasing reliance on SCD therapy. -
Technological Advancements and Smart Devices
The integration of digital health technologies into SCD systems is a defining trend for 2026. Next-generation devices are incorporating real-time pressure monitoring, wireless connectivity (IoT), and mobile app integration to enable remote patient monitoring and adherence tracking. These “smart SCDs” improve clinical outcomes by ensuring consistent use and allowing clinicians to adjust therapy based on patient data. Companies like Medline, 3M, and KCI (a part of 3M) are leading in innovation, with FDA-cleared devices featuring enhanced compliance analytics. -
Expansion in Home Healthcare Settings
The shift toward value-based care and shorter hospital stays is accelerating the adoption of SCDs in home and outpatient environments. By 2026, the home-use segment is expected to grow at a CAGR exceeding 8%, driven by patient preference for convenience, cost containment efforts, and telehealth infrastructure improvements. Portable, user-friendly SCD units with simplified interfaces are gaining traction, supported by reimbursement policies in the U.S. and parts of Europe. -
Regulatory and Reimbursement Landscape
Regulatory scrutiny around medical device safety and efficacy remains high. In 2026, FDA and EMA guidelines are expected to emphasize device interoperability and cybersecurity, especially for connected SCD systems. Concurrently, favorable reimbursement codes (e.g., CPT code 97016 in the U.S.) and inclusion in VTE prevention bundles under CMS initiatives will support market growth. However, pricing pressures in public healthcare systems may constrain margins in regions like Western Europe and Asia-Pacific. -
Emerging Markets and Regional Growth
Asia-Pacific, Latin America, and the Middle East are emerging as high-growth regions due to improving healthcare infrastructure, rising surgical volumes, and growing awareness of VTE prophylaxis. China and India, in particular, are investing in hospital modernization, creating opportunities for SCD manufacturers. Localized manufacturing and partnerships with regional distributors will be key strategies for market penetration. -
Competitive Landscape and Market Consolidation
The SCD market is moderately consolidated, with major players focusing on product differentiation and strategic acquisitions. By 2026, companies are likely to expand their portfolios through mergers or collaborations—especially between device makers and digital health platforms. Increased competition from low-cost manufacturers in Asia may challenge pricing but will also democratize access.
In conclusion, the 2026 SCD market reflects a convergence of clinical necessity, technological innovation, and healthcare transformation. Stakeholders who prioritize patient-centric design, data integration, and global accessibility are best positioned to lead in this evolving landscape.

Common Pitfalls When Sourcing SCD (Sequential Compression Device) – Quality and Intellectual Property Risks
Sourcing Sequential Compression Devices (SCDs) requires careful evaluation to ensure patient safety, clinical efficacy, and legal compliance. Overlooking critical quality and intellectual property (IP) aspects can lead to significant risks, including product failure, regulatory non-compliance, and legal liabilities. Below are common pitfalls to avoid.
Poor Manufacturing Quality and Regulatory Non-Compliance
One of the most frequent pitfalls is sourcing SCDs from manufacturers that do not adhere to stringent medical device quality standards. Low-cost suppliers may lack certifications such as ISO 13485 or FDA registration, increasing the risk of substandard materials, inconsistent performance, and device malfunction. Devices that fail to meet regulatory requirements in your target market (e.g., FDA 510(k), CE Marking under MDR) can result in import bans, recalls, or liability in case of patient injury.
Inadequate Clinical Validation and Performance Testing
Many third-party or private-label SCDs lack robust clinical validation. Buyers may assume equivalent performance to established brands like Stryker or Medline, but without independent testing data, there’s no guarantee that compression patterns, pressure delivery, or cycle timing meet therapeutic standards. This can compromise patient outcomes, especially in high-risk populations such as those at risk for deep vein thrombosis (DVT).
Counterfeit or Clone Devices Infringing IP
A major IP risk involves sourcing devices that mimic patented designs, software algorithms, or branding of original equipment manufacturers (OEMs). Some suppliers offer “compatible” or “equivalent” SCDs that may infringe on design patents, utility patents, or registered trademarks. Using or distributing such devices can expose your organization to cease-and-desist orders, litigation, and financial damages. Always verify that the supplier holds legitimate rights or licenses to the technology.
Lack of Transparency in Supply Chain and Component Sourcing
Opaque supply chains increase the risk of unknowingly procuring devices containing counterfeit parts, restricted substances (e.g., RoHS or REACH non-compliant materials), or components from sanctioned regions. This undermines quality assurance and can lead to regulatory scrutiny, especially in markets with strict traceability requirements.
Insufficient Documentation and Technical Support
Low-cost suppliers may fail to provide comprehensive technical documentation, including software validation reports, electrical safety certifications (e.g., IEC 60601), or instructions for use in required languages. This lack of documentation complicates regulatory submissions and increases the burden on your internal compliance team. Additionally, poor post-market support can delay troubleshooting and impact patient care.
Overlooking Software and Firmware IP Risks
Modern SCDs often include embedded software for controlling compression sequences and monitoring therapy. Unauthorized replication or modification of firmware can violate copyright or software patents. Ensure that any software included with the device is properly licensed and does not incorporate open-source components in violation of their licenses (e.g., GPL).
Failure to Conduct Supplier Audits and Due Diligence
Relying solely on product samples or supplier claims without conducting onsite audits or requesting proof of compliance increases exposure to quality and IP risks. Due diligence should include reviewing design history files (DHF), risk management files (ISO 14971), and freedom-to-operate (FTO) analyses to confirm the device does not infringe existing IP.
By proactively addressing these pitfalls—prioritizing certified quality management systems, validating clinical performance, and ensuring IP integrity—organizations can mitigate risks and source SCDs that are safe, effective, and legally compliant.
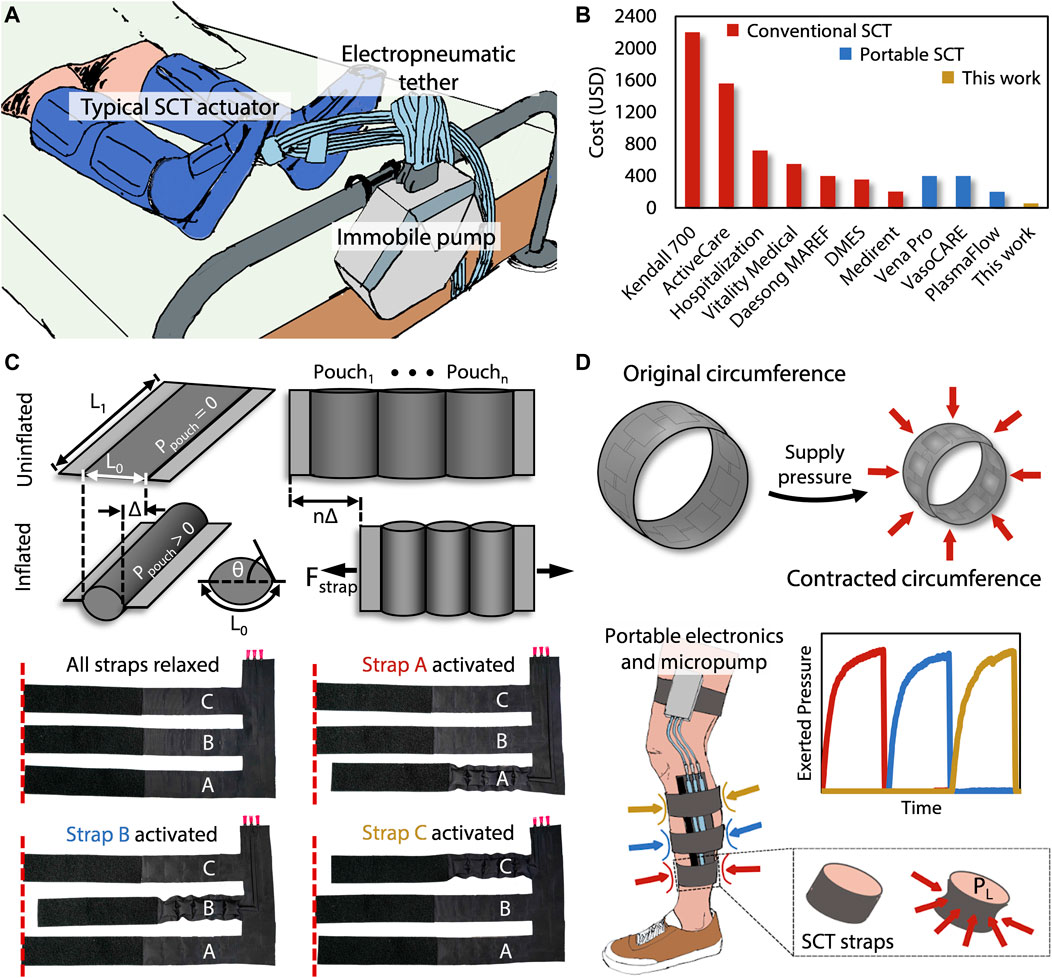
Logistics & Compliance Guide for SCD™ Sequential Compression Device
This guide outlines the essential logistics and compliance considerations for the handling, distribution, use, and maintenance of the SCD™ (Sequential Compression Device) to ensure patient safety, regulatory adherence, and operational efficiency.
Device Overview and Intended Use
The SCD™ Sequential Compression Device is a pneumatic compression system designed to help prevent venous thromboembolism (VTE), including deep vein thrombosis (DVT) and pulmonary embolism (PE), in at-risk patients. It is indicated for use in hospital and clinical settings for adult patients undergoing surgery or those who are immobile due to illness or injury.
Regulatory Compliance
All SCD™ devices and accessories must comply with applicable medical device regulations, including but not limited to:
- FDA 510(k) Clearance: The device is cleared by the U.S. Food and Drug Administration under K number(s) as a Class II medical device.
- CE Marking: Complies with the European Union Medical Device Regulation (MDR) 2017/745, indicating conformity with health, safety, and environmental protection standards.
- ISO Standards: Meets ISO 13485 (Quality Management for Medical Devices) and ISO 14971 (Risk Management for Medical Devices).
- Labeling Requirements: Device labeling must include UDI (Unique Device Identification), manufacturer information, intended use, contraindications, and single-use or reusable status as applicable.
Healthcare facilities and distributors must ensure that only legally marketed and up-to-date versions of the SCD™ system are in use.
Storage and Handling
Proper storage and handling are critical to maintaining device integrity and sterility:
- Storage Conditions: Store in a dry, cool environment at temperatures between 15°C and 30°C (59°F to 86°F). Avoid direct sunlight and exposure to extreme temperatures or humidity.
- Packaging Integrity: Inspect packaging for damage prior to use. Do not use if packaging is torn, punctured, or otherwise compromised.
- Expiration Dates: Check expiration dates on disposables (e.g., SCD sleeves). Do not use expired products.
- Segregation: Store sterile disposables separately from non-sterile items and chemicals.
Distribution and Inventory Management
Effective logistics ensure timely availability and traceability:
- Supply Chain Controls: Partner with authorized distributors only. Verify supplier credentials and maintain distribution records.
- Inventory Tracking: Utilize UDI and barcode scanning to monitor stock levels, expiration dates, and product recalls.
- Cold Chain (if applicable): Not required for standard SCD™ components, but confirm with manufacturer if new variants require special handling.
- Recall Preparedness: Establish a procedure for rapid response to product recalls or field safety notices, including notification systems and quarantine protocols.
Clinical Use and Operator Training
Compliance during clinical use is essential for patient safety:
- Prescriber Authorization: Use only under the order of a licensed healthcare provider.
- Staff Training: Ensure clinicians are trained on proper device setup, patient assessment, sleeve application, and troubleshooting. Training records must be maintained.
- Patient Assessment: Confirm absence of contraindications (e.g., congestive heart failure, active DVT, severe arterial insufficiency) before use.
- Single-Use vs. Reusable Components: Clearly differentiate between single-use sleeves (e.g., SCD Express™) and reusable components (e.g., SCD controller). Never reuse single-use items.
Maintenance and Servicing
For reusable components such as the SCD™ controller:
- Scheduled Maintenance: Follow manufacturer-recommended servicing intervals.
- Calibration and Testing: Perform regular functional checks and calibration as per the service manual.
- Repair Authorization: Only qualified technicians or authorized service providers should perform repairs.
- Documentation: Maintain logs of all maintenance, repairs, and inspections.
Infection Control and Bioburden Management
- Reusable Components: Clean and disinfect the SCD™ controller and tubing between patients according to the manufacturer’s instructions and facility infection control policy.
- Disposable Sleeves: Use a new sleeve for each patient. Dispose of used sleeves in accordance with biomedical waste regulations.
- Cross-Contamination Prevention: Never share sleeves between patients, even with cleaning.
Documentation and Recordkeeping
Maintain comprehensive records to support compliance audits:
- Device usage logs
- Maintenance and repair records
- Staff training documentation
- Inventory and expiration tracking
- Adverse event reports (e.g., via FDA MedWatch or equivalent)
- Recall and field correction actions
Disposal and Environmental Compliance
- Disposables: Dispose of single-use sleeves and connectors as regulated medical waste according to local, state, and federal guidelines.
- Electronics: Recycle controllers and power units through certified e-waste programs.
- Hazardous Materials: Confirm absence of hazardous substances (e.g., lead, mercury); comply with RoHS and WEEE directives where applicable.
Reporting and Incident Management
- Adverse Events: Report any device-related injuries, malfunctions, or near misses to the appropriate regulatory body (e.g., FDA, EUDAMED) and the manufacturer.
- Root Cause Analysis: Investigate incidents to prevent recurrence.
- Manufacturer Notifications: Promptly communicate field safety notices or design changes to end users.
By adhering to this logistics and compliance guide, healthcare providers and distributors can ensure the safe, effective, and lawful use of the SCD™ Sequential Compression Device across the care continuum.
Conclusion for Sourcing SCD (Sequential Compression Device):
In conclusion, sourcing a Sequential Compression Device (SCD) requires a comprehensive evaluation of clinical needs, patient safety, device efficacy, cost-effectiveness, and vendor reliability. After careful assessment of available options, it is evident that investing in high-quality, clinically proven SCD systems enhances patient outcomes by effectively reducing the risk of deep vein thrombosis (DVT) and venous thromboembolism (VTE), particularly in postoperative and immobile patients. Key factors such as ease of use, compatibility with existing workflows, maintenance requirements, and training support must be prioritized during procurement.
Furthermore, engaging with reputable suppliers offering strong technical support, warranty coverage, and responsive service ensures long-term operational efficiency and device reliability. By aligning sourcing decisions with both clinical guidelines and organizational objectives, healthcare facilities can optimize patient care while maintaining cost control and resource efficiency. Ultimately, a well-considered SCD sourcing strategy contributes significantly to improved patient safety and overall quality of care.