The global sleep diagnostics (SCD) devices market is witnessing robust growth, driven by rising awareness of sleep disorders such as obstructive sleep apnea, increasing prevalence of chronic conditions like obesity and cardiovascular diseases, and a growing preference for home-based testing over traditional in-lab polysomnography. According to a report by Mordor Intelligence, the global sleep apnea devices market—of which SCD machines are a critical component—is projected to grow at a CAGR of over 8.5% from 2023 to 2028. This expansion is further fueled by technological advancements, including wireless connectivity, improved data analytics, and compact, user-friendly designs that support remote monitoring and early diagnosis. With Grand View Research estimating that the global sleep disorder treatment market will exceed USD 120 billion by 2030, manufacturers are increasingly focusing on developing at-home SCD solutions that offer clinical accuracy while enhancing patient comfort and accessibility. As demand surges, several key players have emerged as innovators in the space, delivering reliable, data-rich machines tailored for home use—reshaping how sleep health is assessed outside clinical settings.
Top 6 Scd Machine For Home Manufacturers 2026
(Ranked by Factory Capability & Trust Score)
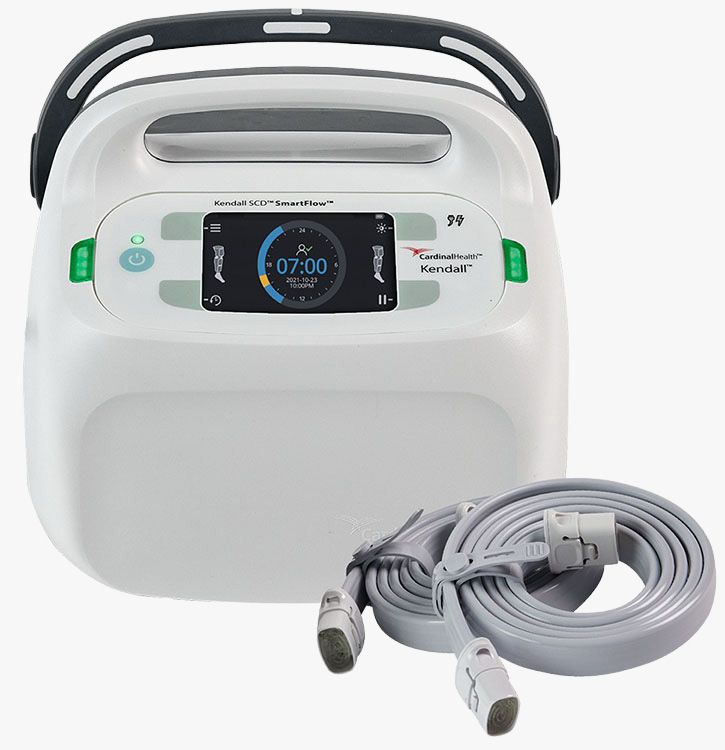
#1 Kendall SCD SmartFlow™ Controller
Domain Est. 1996
Website: cardinalhealth.com
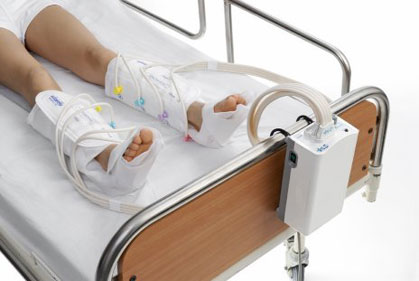
Key Highlights: The SmartFlow™ Compression System applies intermittent pneumatic compression to help prevent deep vein thrombosis….
#2 SCD Machines (Sequential Compression Devices)
Domain Est. 1996
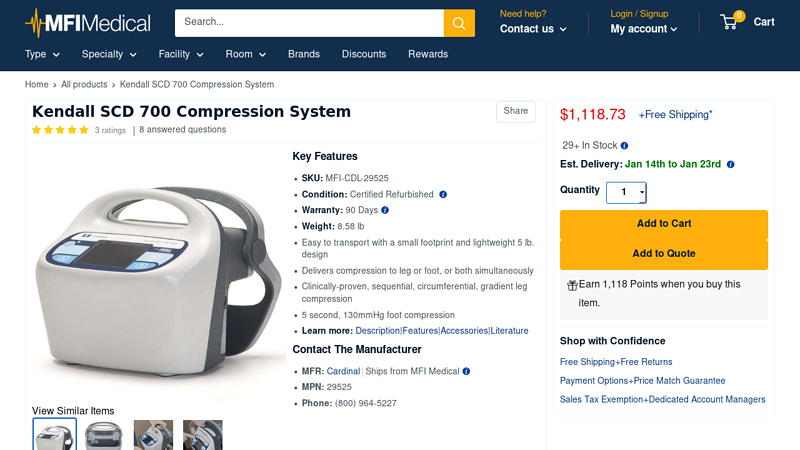
#3 Kendall SCD 700 Compression System
Domain Est. 1998
Website: mfimedical.com
Key Highlights: In stock Rating 5.0 (3) The Kendall SCD 700 compression system delivers clinically proven sequential, gradient, circumferential compression (to the leg, foot or both simultaneous…
#4 SCDS DVT Prevention
Domain Est. 2009
Website: compressionsolutions.us
Key Highlights: The Triple Play VT DVT-EZ Home Care Kit contains one portable Sequential Compression Device (SCDS) DVT prevention pump and two calf sleeves….
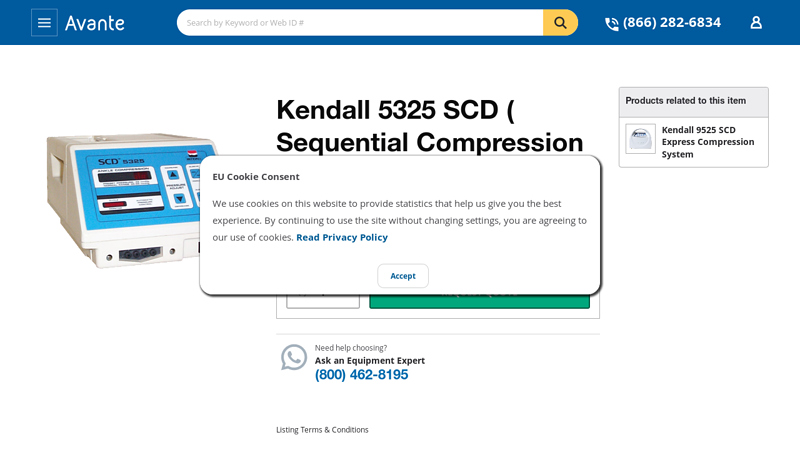
#5 Kendall 5325 SCD ( Sequential Compression Device )
Domain Est. 2017
#6 Buy Covidien Kendall SCD 700 DVT Pump Online at best price
Domain Est. 2003
Website: hospitalstore.com
Key Highlights: The Kendall SCD Smart Compression System is a clinically proven intermittent pneumatic compression (IPC) device designed to increase venous ……
Expert Sourcing Insights for Scd Machine For Home

2026 Market Trends for SCD Machine For Home
The market for Stem Cell-Derived (SCD) machines for home use is poised for significant transformation by 2026. As regenerative medicine advances and consumer interest in personalized health solutions grows, home-based SCD technologies are expected to become more accessible, efficient, and integrated into daily wellness routines. This analysis explores the key trends shaping the SCD machine for home market in 2026.
Technological Advancements and Miniaturization
By 2026, expect rapid progress in the miniaturization and automation of SCD machines. Innovations in microfluidics, AI-driven cell processing, and closed-loop bioreactor systems will allow compact, user-friendly devices to operate safely in home environments. These systems will leverage artificial intelligence to monitor cell growth, optimize culture conditions, and alert users to anomalies, reducing the need for professional oversight.
Rising Demand for Personalized Regenerative Therapies
Growing awareness of anti-aging, immune support, and tissue regeneration will fuel consumer demand for at-home regenerative solutions. SCD machines will cater to individuals seeking autologous (self-derived) cell therapies for skin rejuvenation, joint health, and overall vitality. Market drivers include aging populations in North America, Europe, and parts of Asia, where disposable income supports investment in advanced wellness technologies.
Regulatory Evolution and Safety Standards
Regulatory frameworks are expected to adapt by 2026, with agencies like the FDA and EMA introducing clearer guidelines for home-use regenerative devices. Emphasis will be placed on sterility, traceability, and data security. Manufacturers will need to comply with stringent safety certifications, ensuring that home SCD machines meet clinical-grade standards despite their consumer orientation.
Integration with Digital Health Platforms
SCD machines will increasingly connect to broader digital health ecosystems. By 2026, most devices will sync with mobile apps, wearable biometrics, and telehealth services. Users will track cell viability, receive personalized therapy recommendations, and share anonymized data with healthcare providers. This integration will enhance treatment efficacy and build trust in home-based regenerative medicine.
Cost Reduction and Market Expansion
Initial high costs have limited adoption, but by 2026, economies of scale and production improvements will make SCD machines more affordable. Subscription models offering device leasing, reagent refills, and remote monitoring will lower entry barriers. This will open the market to middle-income consumers, particularly in emerging economies like South Korea, Japan, and parts of Latin America.
Ethical and Social Considerations
As home SCD use becomes mainstream, ethical debates around DIY biotechnology will intensify. Concerns about unregulated self-treatment, genetic modification, and equitable access will prompt public discourse. Industry leaders and policymakers will need to collaborate on educational initiatives and responsible innovation frameworks.
Conclusion
By 2026, the SCD machine for home market will be characterized by technological sophistication, increased accessibility, and integration into personalized healthcare. While challenges remain, the convergence of biotechnology, AI, and consumer wellness trends positions this sector for substantial growth, transforming how individuals approach long-term health and regeneration from the comfort of their homes.

Common Pitfalls When Sourcing SCD Machines for Home Use (Quality and Intellectual Property)

Logistics & Compliance Guide for SCD Machine For Home
Product Classification and Regulatory Requirements
Before shipping or selling an SCD (Structured Carbon Dioxide) machine for home use, ensure the product is correctly classified under international and local regulatory frameworks. These devices may fall under medical, wellness, or consumer electronics categories, depending on their intended use.
- FDA (U.S. Food and Drug Administration): If marketed for therapeutic or medical purposes, FDA clearance or registration may be required. Even wellness claims may trigger regulatory scrutiny.
- CE Marking (Europe): Required for sale in the European Economic Area. Compliance with directives such as the Low Voltage Directive (LVD), Electromagnetic Compatibility (EMC), and potentially the Medical Devices Regulation (MDR) must be evaluated.
- Health Canada: If making health-related claims, the device may require review under the Medical Devices Regulations.
- Other Markets: Check local health and safety standards in target countries (e.g., TGA in Australia, PMDA in Japan).
Consult a regulatory compliance expert to determine the appropriate classification based on labeling, marketing claims, and functionality.
Import and Export Documentation
Proper documentation is essential to avoid customs delays and ensure legal entry into target markets.
- Commercial Invoice: Must include product description, value, Harmonized System (HS) code, country of origin, and buyer/seller details.
- Packing List: Details weight, dimensions, and packaging for each shipment.
- Certificate of Origin: May be required for tariff determination or trade agreements.
- Bill of Lading or Air Waybill: Legal contract between shipper and carrier.
- Compliance Certificates: Include CE Declaration of Conformity, FDA registration (if applicable), RoHS, and other relevant certifications.
Ensure the HS code (e.g., 8543.70 for electrical therapeutic devices) is accurately assigned to determine tariffs and restrictions.
Shipping and Transportation
SCD machines are typically low-volume, high-value items suitable for express courier or air freight.
- Packaging: Use durable, shock-resistant materials with clear labeling (fragile, orientation arrows). Include moisture protection if necessary.
- Battery Regulations: If the device contains lithium batteries, comply with IATA Dangerous Goods Regulations for air transport (UN3480/3481).
- Temperature and Humidity: Store and ship within manufacturer-specified environmental conditions to prevent damage.
- Insurance: Recommend full-value insurance due to product value and sensitivity.
Partner with logistics providers experienced in shipping regulated consumer health devices.
Customs Clearance and Duties
Customs clearance times vary by country. Be prepared for inspections, especially with medical or electronic devices.
- Duties and Taxes: Research applicable import duties, VAT, or GST in destination countries. Duties may range from 0% to 15% depending on classification and trade agreements.
- Customs Brokers: Use licensed brokers in key markets to ensure compliance and expedite clearance.
- Labeling Requirements: Ensure outer packaging includes compliant labeling in the local language (e.g., French in Canada, German in Germany).
Delays may occur if documentation is incomplete or if the device is flagged for regulatory review.
Warranty, Returns, and After-Sales Support
Establish a clear policy for international returns and customer support.
- Warranty Compliance: Comply with local consumer protection laws (e.g., 2-year warranty in the EU under the Consumer Rights Directive).
- Return Logistics: Define return shipping procedures, especially for cross-border returns. Consider regional service centers to reduce return shipping costs.
- Technical Support: Offer multilingual support and comply with local data privacy laws (e.g., GDPR) when handling customer information.
Environmental and Safety Compliance
Ensure the product meets environmental standards throughout its lifecycle.
- RoHS (Restriction of Hazardous Substances): Required in the EU and many other regions for electronic devices.
- WEEE (Waste Electrical and Electronic Equipment): Registration may be required for take-back and recycling programs in Europe.
- Energy Efficiency: Comply with local energy standards such as ENERGY STAR (U.S.) or Ecodesign (EU).
Final Recommendations
- Conduct a full compliance audit before launching in any new market.
- Maintain up-to-date technical files and conformity documentation.
- Work with legal and logistics partners familiar with health and wellness devices.
- Monitor regulatory changes, especially in fast-evolving wellness technology sectors.
By following this guide, businesses can ensure smooth logistics operations and full compliance when distributing SCD machines for home use worldwide.
Conclusion for Sourcing an SCD Machine for Home Use
After thorough research and consideration of various factors such as cost, space, maintenance, and long-term benefits, sourcing an SCD (Sickle Cell Disease) monitoring or support machine for home use can be a practical and beneficial decision for individuals managing sickle cell conditions. While there may not be a single “SCD machine” that cures the disease, devices such as pulse oximeters, hydration monitors, pain management tools (e.g., TENS units), respiratory support equipment, or even home-based transcranial Doppler (TCD) screening units (where available) can significantly enhance patient comfort, early detection of complications, and overall quality of life.
Key advantages of having such equipment at home include:
– Timely monitoring of vital signs and early warning of complications like acute chest syndrome or stroke risk.
– Reduced need for frequent hospital visits, lowering healthcare costs and stress.
– Empowerment of patients and caregivers to actively participate in disease management.
– Improved adherence to treatment plans through consistent tracking.
However, it’s essential to consult with a hematologist or healthcare provider before purchasing any device to ensure it is clinically appropriate, accurate, and integrated into the individual’s care plan. Additionally, factors like device reliability, ease of use, data connectivity, and insurance coverage should be carefully evaluated.
In conclusion, sourcing the right medical devices for home use in SCD management is a worthwhile investment in proactive, patient-centered care—provided it is done with medical guidance and realistic expectations. With the right tools and support, individuals with sickle cell disease can achieve greater comfort, safety, and independence in their daily lives.