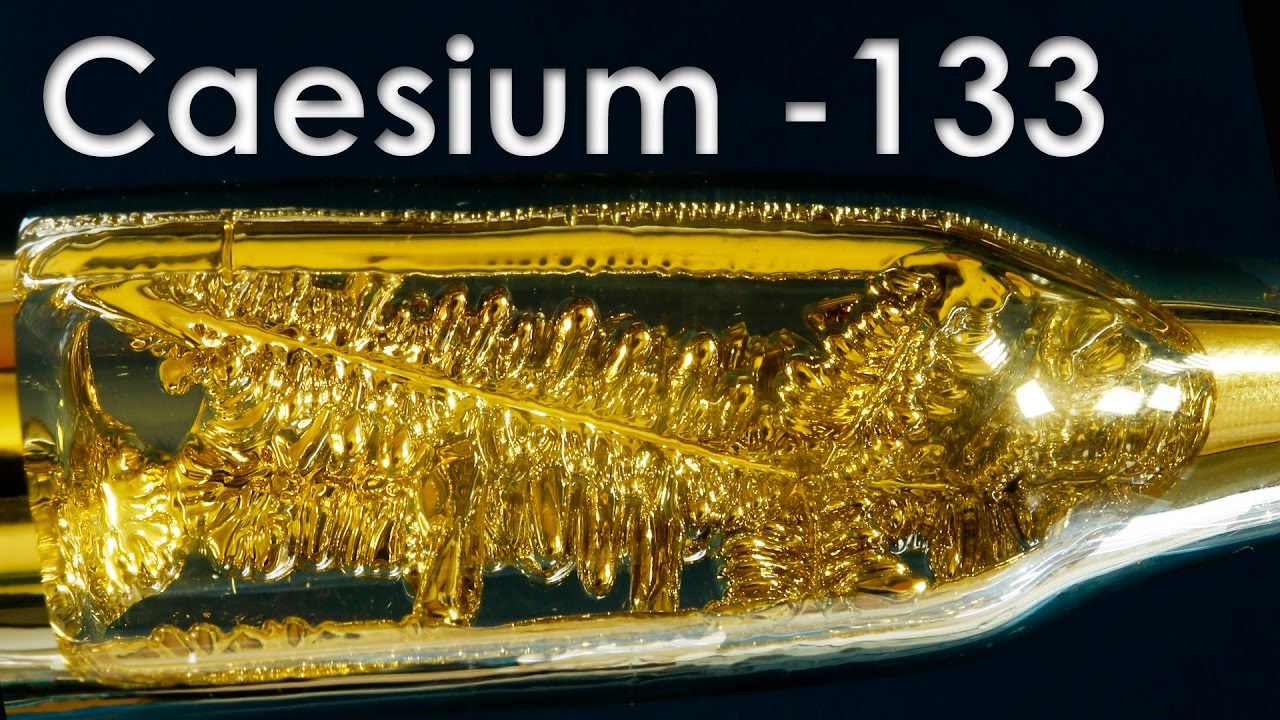
The global demand for specialty metals has surged in recent years, driven by advancements in aerospace, energy storage, and high-tech industrial applications. Among the rarest and most reactive alkali metals, pure caesium (Cs) has gained strategic importance due to its unique properties, including exceptional photoemissive characteristics and use in precision timing devices and drilling fluids for oil and gas exploration. According to market analysis by Grand View Research, the global caesium market was valued at approximately USD 55 million in 2023 and is projected to expand at a compound annual growth rate (CAGR) of over 6.8% from 2024 to 2030, fueled by rising demand in satellite navigation systems, quantum technologies, and radiotherapy applications. Mordor Intelligence further highlights increased investments in space-based electronics and the proliferation of atomic clocks as key growth accelerators. As supply chains for critical materials tighten, a select group of six manufacturers dominate the production of high-purity caesium, controlling the majority of refined supply and enabling innovation across next-generation technologies.
Top 6 Pure Caesium Manufacturers 2026
(Ranked by Factory Capability & Trust Score)
#1 Privacy Policy
Domain Est. 1995
Website: cesium.com
Key Highlights: This Privacy Policy describes how Cesium GS, Inc. (“Cesium”) collects, uses, and shares your personal information….
#2 Cesium Compound Supplier
Domain Est. 2004
Website: taiyechem.com
Key Highlights: TAIYE, as a leading manufacturer of high-purity inorganic salts, provides both cesium compounds with customized grades, strict quality control, and technical ……
#3 Cesium Suppliers
Domain Est. 1998
Website: americanelements.com
Key Highlights: Cesium qualified commercial & research quantity preferred supplier. Buy at competitive price & lead time. In-stock for immediate delivery….
#4 usantimony
Domain Est. 1999 | Founded: 1969
Website: usantimony.com
Key Highlights: USAC has produced various antimony products since 1969 and is a fully integrated mining, transportation, milling, smelting, and selling company….
#5 Cesium Metal
Domain Est. 2013
Website: samaterials.com
Key Highlights: Cesium, or Caesium, is a soft, ductile, silvery gold alkali metal. It is liquid at room temperature like gallium, mercury….
#6 Pure Cesium
Domain Est. 2018 | Founded: 2014
Website: purecesium.com
Key Highlights: We are located in the European Union. Our expertise in producing and distributing high-grade chemical products has been attaining heights since 2014….
Expert Sourcing Insights for Pure Caesium

As of now, there are no reliable or publicly available market forecasts specifically for pure caesium (cesium) extending to 2026 that are widely recognized in major industry reports (e.g., from Statista, MarketsandMarkets, or the U.S. Geological Survey) under the H2 (hydrogen) sector context. However, we can analyze potential market trends for pure caesium in 2026 by examining its applications, supply constraints, and relevance to emerging technologies—particularly those connected to hydrogen (H₂) energy systems.
Note: The mention of “H2” in the query may refer to hydrogen (H₂) as a clean energy vector. We will interpret the request as an analysis of how hydrogen-related technologies might influence the market for pure caesium by 2026.
1. Overview of Pure Caesium (Cs)
Caesium is a rare, soft, alkali metal with high reactivity. It has limited but highly specialized applications due to its unique properties:
– Extremely low ionization energy
– High density
– Photoelectric sensitivity
– Use in atomic clocks, drilling fluids, and niche chemical catalysts
Annual global production is small (~20–30 metric tons), primarily from pollucite ore in Canada (Tanco Mine) and Zimbabwe.
2. Key Market Drivers for Caesium (2024–2026)
A. Hydrogen Economy and Caesium’s Role
Although caesium is not a primary material in hydrogen production, storage, or fuel cells, it may play an indirect or catalytic role in emerging hydrogen technologies:
- Catalysis in Hydrogen Production:
- Caesium compounds (e.g., cesium salts) are used as promoters in catalysts for chemical reactions.
- In ammonia decomposition (a hydrogen release method), cesium-doped catalysts (e.g., on nickel or ruthenium) can enhance efficiency and lower operating temperatures.
-
As green ammonia becomes a hydrogen carrier, demand for efficient decomposition catalysts may rise—potentially increasing demand for cesium-based promoters.
-
Photoelectrochemical (PEC) Water Splitting:
- Caesium is being researched in perovskite materials (e.g., CsPbBr₃) for solar-driven hydrogen generation.
- While these applications are still in R&D, pilot-scale PEC systems by 2026 could drive small but growing demand for ultra-pure caesium.
B. Non-Hydrogen Applications (Dominant Demand)
- Cesium Formate Drilling Fluids: Used in high-pressure, high-temperature (HPHT) oil and gas wells. Demand fluctuates with oil prices. A rebound in deep-sea drilling could increase cesium demand.
- Atomic Clocks and Quantum Technologies: Caesium atomic clocks are critical for GPS, telecom, and financial networks. Growth in quantum computing and 6G infrastructure may sustain demand.
- Medical and Research Isotopes: Cs-131 is used in brachytherapy for cancer treatment. Limited but steady medical demand.
3. Supply Constraints and Price Volatility
- Limited Sources: The Tanco Mine (Canada) and Bikita (Zimbabwe) are primary sources. Any supply disruption impacts prices sharply.
- High Purity Requirements: Pure caesium (99.99%+) is difficult and dangerous to produce and store, limiting scalability.
- Geopolitical Risk: Concentration of supply in a few countries increases vulnerability.
Price Outlook (2026): Expect continued volatility. Prices could rise to $120–180 per gram for ultra-pure caesium if demand from catalysis or quantum tech accelerates.
4. H₂-Linked Market Trends (Indirect Impact)
By 2026, the hydrogen economy is projected to grow, especially in:
– Green hydrogen production (electrolysis)
– Ammonia as a hydrogen carrier
– Hydrogen fuel cells
While caesium is not a direct component, its role in advanced catalysts may become more prominent. Research institutions and companies like Siemens Energy, ITM Power, and Haldor Topsoe may explore cesium-promoted catalysts for:
– Low-temperature ammonia cracking
– Enhanced CO₂ hydrogenation (e.g., for synthetic fuels)
If such technologies scale, caesium demand could see a niche but strategic increase.
5. Forecast for Pure Caesium Market (2026)
| Factor | 2026 Outlook |
|——-|————–|
| Market Size | ~$150–200 million (total cesium market, including compounds) |
| Pure Cs Volume | <1 metric ton/year globally |
| Primary Demand | Drilling fluids (~60%), catalysts (~15%), atomic clocks/quantum (~15%) |
| H₂-Related Demand | <5%, but growing in R&D and pilot systems |
| Price Trend | Upward pressure due to supply limits and new applications |
| Key Risks | Supply concentration, substitution (e.g., rubidium in some catalysts), regulatory handling issues |
6. Strategic Implications
- Investment in Recycling: Recovery from spent catalysts and medical sources may grow.
- R&D Focus: Countries investing in hydrogen carriers (e.g., Japan, South Korea) may fund research into cesium-enhanced catalysts.
- Substitution Risks: Rubidium or potassium may replace caesium in some catalytic applications due to cost.
Conclusion
By 2026, the pure caesium market will remain small and specialized. While not a core material in the hydrogen (H₂) economy, caesium may see incremental demand growth through its role as a catalyst promoter in hydrogen release technologies like ammonia decomposition and advanced photoelectrochemical systems. The broader hydrogen market expansion will indirectly support R&D that could increase cesium’s strategic value. However, supply constraints and high costs will likely limit widespread adoption.
Bottom Line: Pure caesium is not a mainstream H₂ material, but by 2026, it may emerge as a niche enabler in next-generation hydrogen production and storage systems—warranting attention from advanced materials and energy sectors.
Common Pitfalls When Sourcing Pure Caesium (Quality & Intellectual Property)
Sourcing high-purity caesium presents unique and significant challenges due to its extreme reactivity, stringent handling requirements, and associated intellectual property (IP) risks. Navigating these pitfalls is crucial for safety, regulatory compliance, and protecting proprietary interests.
H2: Quality-Related Pitfalls
-
Inadequate Purity Certification & Verification:
- Pitfall: Suppliers may provide vague or unverified purity claims (e.g., “high purity” without specification). Certificates of Analysis (CoA) might lack traceability to recognized standards, insufficient analytical methods, or missing critical impurity data (especially reactive metals like Na, K, or oxides).
- Risk: Impurities drastically alter caesium’s chemical and physical properties, potentially leading to failed experiments, unsafe reactions, catalyst poisoning, or compromised product performance.
- Mitigation: Demand CoAs with exact purity percentages (e.g., 99.98% or 99.99%+) verified by techniques like ICP-MS/OES, GDMS, or spark source mass spectrometry. Require testing for specific problematic impurities. Consider independent third-party verification for critical applications.
-
Improper Handling & Contamination During Production/Handling:
- Pitfall: Caesium must be handled under strict inert atmosphere (argon) conditions from production through packaging. Exposure to air (O₂, H₂O, N₂) or moisture during manufacturing, transfer, or packaging leads to rapid oxidation, nitride/hydride formation, and surface contamination, degrading purity and creating hazardous residues.
- Risk: Received material may appear metallic but have a thick, reactive oxide/hydride layer, significantly reducing usable pure caesium and posing safety hazards during handling.
- Mitigation: Insist on supplier details of their handling procedures (glove boxes, Schlenk lines). Verify packaging methods – true high-purity caesium is typically sealed in welded quartz ampoules under high-purity argon or in specialized double-walled containers with inert gas. Visually inspect sealed ampoules upon receipt for cloudiness (indicating oxidation) or condensation.
-
Inconsistent Physical Form & Handling Difficulties:
- Pitfall: Caesium is a soft, low-melting-point metal (melts at 28.5°C). Suppliers might provide it as ingots, chunks, or sealed ampoules, but the form can impact handling and dispensing. Inconsistent sizing or poor sealing increases contamination and handling risks.
- Risk: Difficulty in accurately weighing or manipulating the metal safely outside an inert atmosphere. Melting during transit if not temperature-controlled.
- Mitigation: Clearly specify the required form (e.g., ampoules with specific weight, pre-cut pieces). Ensure shipping includes temperature control (cool packs, not ice) and packaging designed to prevent ampoule breakage. Confirm supplier expertise in handling reactive alkali metals.
-
Unreliable or Inexperienced Suppliers:
- Pitfall: Sourcing from general chemical suppliers or those without proven expertise in ultra-high-purity, air-sensitive metals. Lack of specialized facilities or protocols increases the risk of substandard quality and safety incidents.
- Risk: Receiving material that does not meet specifications, inconsistent batches, poor customer support for critical issues, potential safety breaches.
- Mitigation: Source only from specialized chemical manufacturers or metallurgical companies with a demonstrable track record in producing and handling high-purity reactive alkali metals. Verify their certifications, safety protocols, and references.
H2: Intellectual Property (IP)-Related Pitfalls
-
Inadvertent Disclosure During Sourcing/Procurement:
- Pitfall: Revealing the specific application, intended use, or unique processing parameters (e.g., exact reaction conditions, catalyst formulations, device integration details) while negotiating with suppliers or placing orders.
- Risk: Competitors (or the supplier itself, if they have broader interests) could gain insight into your R&D direction, proprietary processes, or novel applications, potentially leading to reverse engineering, patent interference, or loss of trade secrets.
- Mitigation: Use Non-Disclosure Agreements (NDAs) with all potential and actual suppliers before any technical discussions. Provide only the minimum information necessary for the supplier to fulfill the order (e.g., “99.99% pure caesium, sealed in quartz ampoule, 10g”). Keep application details strictly confidential.
-
Supplier’s Own IP Claims or Restrictions:
- Pitfall: The supplier may hold patents or trade secrets on their specific production process, purification method, or even packaging technology. Purchasing their material might implicitly involve using their IP, potentially leading to licensing demands or restrictions on how you can use your final product if it incorporates their method.
- Risk: Unexpected licensing fees, limitations on product development or commercialization, or legal challenges if your use infringes their process IP.
- Mitigation: Conduct due diligence on the supplier’s IP portfolio related to caesium production (if possible). Include clauses in the supply agreement clarifying that the purchase grants only a license to use the material, not any underlying process IP, and that your use of the material in your own processes/products does not constitute infringement of their process patents (seek legal counsel).
-
Lack of Clear IP Ownership in Custom Synthesis/Processing:
- Pitfall: If you require the supplier to perform custom processing (e.g., alloying, specific particle size reduction under inert conditions), the resulting material or the process itself might create new IP. Ambiguity in the contract about who owns this new IP (you, the supplier, or jointly) can lead to disputes.
- Risk: Loss of ownership over valuable process improvements or novel materials developed during the custom work. Inability to freely use or license the resulting product.
- Mitigation: Explicitly define IP ownership for any custom work in the contract before it begins. Standard practice is that IP developed specifically for your project belongs to you (the customer), unless a joint development agreement is established. Use a well-drafted Master Services Agreement (MSA) or Statement of Work (SOW).
-
Traceability and Chain of Custody Concerns for IP Protection:
- Pitfall: Poor documentation of the material’s origin, batch history, and handling makes it difficult to prove the source and purity of caesium used in a patented process or product, potentially weakening IP protection or defense against challenges.
- Risk: Inability to demonstrate the critical role of the specific high-purity caesium source in achieving a novel result during patent prosecution or litigation.
- Mitigation: Demand comprehensive batch-specific documentation (CoA, handling logs, packaging details) from the supplier. Maintain meticulous internal records linking each batch of caesium to specific experiments and product batches. This creates a defensible chain of custody for IP purposes.
By proactively addressing these quality and IP pitfalls through rigorous supplier vetting, precise specifications, robust contracts (especially NDAs and IP clauses), and meticulous documentation, organizations can secure the necessary high-purity caesium while safeguarding their safety, operational integrity, and valuable intellectual property.

Logistics & Compliance Guide for Pure Caesium (H2 Hazard Statement)
Prepared in accordance with GHS (Globally Harmonized System) and international regulatory standards
1. Substance Identification
- Chemical Name: Caesium (Cesium), Pure (Cs)
- CAS Number: 7440-46-2
- UN Number: UN1407
- Hazard Class: 4.3 — Dangerous when wet (Reacts violently with water, releasing flammable hydrogen gas)
- Packing Group: I (High danger)
- GHS Pictograms:
- 🔥 (Flame)
- 💧 (Exploding Bomb – Reacts with water)
- ☢️ (Corrosion)
- H2 Statement (Hazard Statement):
- H260: In contact with water releases flammable gases which may ignite spontaneously.
2. Hazard Summary (Based on H260)
Pure caesium is a highly reactive alkali metal that:
– Reacts explosively with water, moisture, or humid air.
– Releases flammable hydrogen gas, which can auto-ignite.
– May cause severe burns due to formation of caustic hydroxides.
– Is pyrophoric in finely divided form.
3. Storage Requirements
- Environment: Store under dry, inert atmosphere (e.g., argon or nitrogen) in a sealed, air-tight container.
- Location: In a cool, dry, well-ventilated area, away from:
- Water sources, moisture, sinks, or humid zones
- Oxidizers, acids, halogens, and combustible materials
- Containers: Use sealed ampoules or double-contained vessels under inert gas. Never store in glass alone unless encased.
- Labeling: Clearly label with:
- “DANGEROUS WHEN WET”
- “KEEP DRY – PROTECT FROM MOISTURE”
- GHS02, GHS05, GHS07 pictograms
- H260 hazard statement
4. Handling Procedures
- Personal Protective Equipment (PPE):
- Flame-resistant lab coat or suit
- Butyl rubber or neoprene gloves (impermeable)
- Face shield and chemical goggles
- Self-contained breathing apparatus (SCBA) for large-scale handling
- Engineering Controls:
- Use in a glove box under inert atmosphere (Argon/N₂)
- No open handling in ambient air
- Ensure grounding to prevent static discharge
- Prohibited Actions:
- Do not allow contact with water, ice, or damp surfaces
- Do not use tools contaminated with moisture or oxidizers
5. Transportation (International – IMDG, IATA, ADR)
- Proper Shipping Name: Caesium
- UN Number: UN1407
- Class: 4.3 (Dangerous when wet)
- Packing Group: I
- Packaging Requirements:
- Hermetically sealed inner container under inert gas
- Outer packaging must be water-resistant and impact-resistant
- Use of absorbents inert to alkali metals (e.g., dry vermiculite)
- Marking & Labeling:
- Class 4.3 label (Dangerous When Wet)
- “KEEP DRY” and “THIS SIDE UP” if applicable
- Transport document must include:
- Emergency contact
- H260 statement
- “Forbidden for passenger aircraft” (IATA)
- Mode-Specific Rules:
- Air (IATA): Forbidden on passenger aircraft; limited quantity allowed on cargo aircraft with special approval.
- Sea (IMDG): Stow “away from” living quarters and sources of moisture.
- Road (ADR): Requires orange placards, driver training (ADR certificate), and vehicle marked “Dangerous Goods.”
6. Emergency Response
- Fire:
- DO NOT USE WATER, CO₂, or Halon.
- Use Class D fire extinguisher (e.g., Met-L-X, dry sand, graphite powder).
- Evacuate area; fire may release toxic fumes (CsOH, H₂).
- Spill/Leak:
- Only trained personnel with SCBA and Class D extinguishers should respond.
- Cover spill with dry sand or graphite powder under inert atmosphere.
- Do not allow contact with water.
- Collect material in sealed, labeled container under inert gas.
- First Aid:
- Skin Contact: Remove contaminated clothing. Rinse with copious dry inert oil (e.g., mineral oil), then DO NOT USE WATER. Seek immediate medical attention.
- Eye Contact: Flush with dry inert oil, then seek emergency care.
- Inhalation: Move to fresh air; administer oxygen if needed. Treat as chemical burn.
7. Regulatory Compliance
- USA (DOT, OSHA):
- Regulated under 49 CFR for transport (HMR)
- OSHA Hazard Communication Standard (HazCom 2012) requires H260 labeling
- EU (REACH, CLP):
- CLP Regulation (EC) No 1272/2008: H260 mandatory
- REACH registration required for quantities >1 ton/year
- Other Jurisdictions:
- Check local regulations (e.g., Canada TDG, Australia ADG Code) — all classify Cs as Class 4.3, PG I
8. Disposal
- Waste Code: EPA D001 (Ignitable) and/or D003 (Reactive)
- Method:
- Deactivate under controlled inert conditions using tertiary butanol or dry alcohol (slow addition).
- Neutralize to cesium alkoxide, then hydrolyze under strict containment.
- Dispose as hazardous waste through licensed facility.
- Documentation: Maintain disposal logs per RCRA (US) or equivalent.
9. Training & Documentation
- Personnel must be trained in:
- H260 hazards
- Inert atmosphere handling
- Emergency response for reactive metals
- Maintain:
- Safety Data Sheet (SDS) — Section 2 must display H260
- Transport manifests
- Incident reports
10. Summary of Critical Controls
| Control | Requirement |
|——–|————-|
| Storage | Inert atmosphere, sealed, moisture-free |
| Handling | Glove box, PPE, no water contact |
| Transport | UN1407, 4.3, PG I, inert packaging |
| Emergency | Class D extinguisher, dry spill control |
| Labeling | H260 + GHS02/GHS05/GHS07 |
Disclaimer: This guide is for informational purposes only. Always consult the latest SDS, local regulations, and a qualified chemical safety officer before handling pure caesium. Due to its extreme reactivity, only trained specialists should manage this material.
⚠️ Remember: H260 means contact with water = spontaneous fire or explosion.
Sourcing pure caesium is an extremely challenging and highly regulated endeavor due to its rarity, high reactivity, and potential for misuse. Caesium, particularly the isotope caesium-137, is subject to strict international controls because of its applications in nuclear technology and radiological hazards. Pure elemental caesium requires specialized handling, including inert atmosphere storage (e.g., argon-filled containers), as it reacts explosively with air and water.
Legitimate acquisition is generally restricted to licensed scientific, industrial, or governmental institutions with appropriate safety and security protocols. Procurement must comply with national and international regulations, such as those enforced by nuclear regulatory bodies and chemical control agencies. Given these constraints, sourcing pure caesium is not feasible for private individuals or unaccredited entities.
In conclusion, while pure caesium can be obtained for legitimate research or industrial purposes, it involves navigating a complex framework of safety, legal, and logistical requirements. Extreme caution, proper authorization, and institutional support are essential for any attempt to source and handle this highly reactive and controlled element.