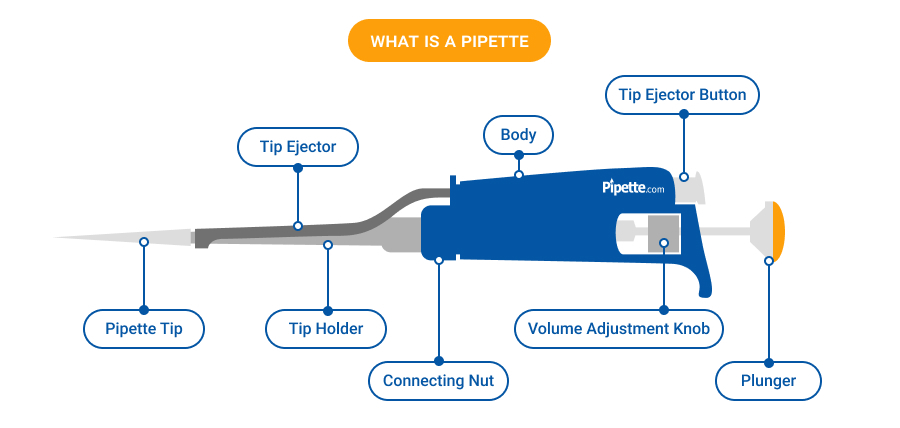
The global laboratory equipment market, driven by increasing R&D investments in pharmaceuticals, biotechnology, and academic research, continues to experience robust growth. According to Mordor Intelligence, the global pipette market was valued at USD 2.7 billion in 2023 and is projected to grow at a CAGR of 6.8% from 2024 to 2029. This expansion is fueled by rising demand for precision liquid handling, automation integration, and stringent quality standards in diagnostic and research laboratories. Grand View Research further notes that advancements in microliter and nanoliter dispensing technologies, coupled with the proliferation of high-throughput screening methods, are reshaping supplier competitiveness. As laboratories prioritize accuracy, ergonomics, and traceability, the need for high-performance pipette sets has intensified—making manufacturer reliability and innovation more critical than ever. In this evolving landscape, identifying leading pipette set manufacturers is key to ensuring lab efficiency and compliance with global standards.
Top 10 Pipette Set Manufacturers 2026
(Ranked by Factory Capability & Trust Score)
#1 Pipette Tips, Pipettors, and Accessories
Domain Est. 1991
Website: corning.com
Key Highlights: Pipette Tips and Accessories. We specialize in manufacturing high quality pipette tips for all major automation platforms, for consistency and reliability in ……
#2 Liquid Handling
Domain Est. 1993
Website: mt.com
Key Highlights: Pipetting 360+ reflects our commitment to providing the very finest pipettes, pipette tips, service, and support….
#3 All Pipettes, Dispensers & Automated Liquid Handlers
Domain Est. 1995
Website: eppendorf.com
Key Highlights: Eppendorf lab pipettes & dispensers are crafted to support you with high accuracy and precision and to allow you to work in an efficient manner….
#4 Pipetting
Domain Est. 1996
Website: sartorius.com
Key Highlights: Our ergonomic pipettes make pipetting easy, accurate and comfortable. See our full range of mechanical and electronic pipettes as well as pipette tips and ……
#5 Starter Set
Domain Est. 1997
Website: pipettes.com
Key Highlights: 4-day delivery 30-day returnsOur pipette starter sets are all-inclusive with pipettes and compatible tips for each volume. Some sets also come with accessories, such as stands and …
#6 Your Reliable Partner For Productive Pipettes
Domain Est. 1997
Website: integra-biosciences.com
Key Highlights: Would you like to improve your pipetting efficiency? We provide you with cutting-edge pipettes, facilitating whatever pipetting task you might have….
#7 Single
Domain Est. 1998
#8 Pipette.com – your go
Domain Est. 2000
Website: pipette.com
Key Highlights: Pipette.com is your go-to scientific supplies company providing high-quality laboratory consumables and equipment for more than 25 years….
#9 Pipette Supplies
Domain Est. 2002
#10 Pipettes and Pipette Tips
Domain Est. 2006
Website: thermofisher.com
Key Highlights: Help improve accuracy and precision with pipetting systems that incorporate years of experience and innovation for more consistent, reproducible results….
Expert Sourcing Insights for Pipette Set

H2: 2026 Market Trends for Pipette Sets
The global pipette set market is poised for significant evolution by 2026, driven by advancements in laboratory automation, increasing demand for precision in life sciences research, and expanding applications in pharmaceuticals, biotechnology, and clinical diagnostics. Several key trends are expected to shape the market landscape in the coming years.
1. Rising Adoption of Electronic and Automated Pipettes
A major trend through 2026 is the shift from manual to electronic and semi-automated pipette systems. Laboratories are increasingly prioritizing accuracy, reproducibility, and ergonomics to reduce human error and repetitive strain injuries. Electronic pipettes with programmable settings, connectivity features (e.g., Bluetooth and cloud integration), and data logging capabilities are gaining traction, especially in high-throughput environments.
2. Integration with Laboratory Information Management Systems (LIMS)
By 2026, interoperability between pipette sets and digital lab infrastructure is expected to be a critical differentiator. Smart pipettes capable of transmitting usage data, calibration records, and performance metrics to LIMS or electronic lab notebooks (ELN) will support compliance with regulatory standards (e.g., GLP, GMP) and enable better traceability in research and production workflows.
3. Focus on Sustainability and Eco-Friendly Designs
Environmental concerns are influencing product development. Manufacturers are responding with pipette sets made from recyclable materials, reduced plastic consumption, and energy-efficient electronic models. Refillable or modular tips and long-life battery systems are also being emphasized to minimize waste and operational costs.
4. Growth in Emerging Markets
The Asia-Pacific region, particularly China, India, and Southeast Asia, is expected to witness the fastest market growth by 2026. This is fueled by increased government and private investments in R&D, expansion of biopharmaceutical manufacturing, and the establishment of new diagnostic and research laboratories.
5. Demand for Multichannel and Adjustable Pipettes
Multichannel pipettes (8-, 12-, and 96-channel) will continue to gain popularity in genomic and high-throughput screening applications. Additionally, adjustable volume pipettes offering wide range spans and fine calibration options will be in demand across academic, industrial, and clinical labs seeking versatility and cost efficiency.
6. Emphasis on Calibration and Service Ecosystems
As precision becomes non-negotiable, manufacturers are expanding their service offerings, including on-site calibration, cloud-based performance monitoring, and predictive maintenance. These value-added services are expected to play a pivotal role in customer retention and brand loyalty.
7. Impact of Technological Innovation
Emerging technologies such as AI-assisted workflow optimization, IoT-enabled devices, and integration with robotic liquid handling platforms will position advanced pipette sets as integral components of smart laboratories by 2026.
In conclusion, the 2026 pipette set market will be characterized by a blend of technological sophistication, user-centric design, and sustainability. Companies that innovate in connectivity, ergonomics, and service integration are likely to lead the market, while end-users will benefit from enhanced accuracy, efficiency, and compliance in their laboratory operations.

Common Pitfalls When Sourcing a Pipette Set: Quality and Intellectual Property Concerns
Sourcing a pipette set—especially for laboratories, research institutions, or manufacturing environments—requires careful evaluation beyond price and availability. Two critical areas where organizations often encounter issues are product quality and intellectual property (IP) risks. Overlooking these can lead to compromised results, equipment failure, legal complications, and reputational damage.
Quality-Related Pitfalls
-
Inconsistent Calibration and Accuracy
Low-cost or uncertified pipettes may lack proper calibration documentation or fail to maintain accuracy across volume ranges. This results in unreliable experimental data, failed quality control tests, and reproducibility issues—especially problematic in regulated environments like pharmaceuticals or clinical diagnostics. -
Poor Material Durability
Inferior materials (e.g., low-grade plastics or metals) can degrade over time, leading to leaks, tip ejection problems, or contamination. Exposure to common solvents or repeated autoclaving may cause warping or cracking, shortening the pipette’s lifespan. -
Lack of Compliance with Standards
Reputable pipette sets should comply with international standards such as ISO 8655 for piston-operated volumetric apparatus. Sourcing non-compliant products risks audit failures during inspections by regulatory bodies (e.g., FDA, ISO auditors). -
Inadequate After-Sales Support and Service
Budget suppliers may offer limited or no repair, recalibration, or technical support. This increases downtime and long-term costs, as damaged pipettes may need full replacement instead of servicing. -
Counterfeit or Misrepresented Products
Some suppliers offer “compatible” or “OEM-style” pipettes that mimic well-known brands but do not meet the same performance standards. These may be counterfeit or reverse-engineered without proper quality control, misleading buyers about their capabilities.
Intellectual Property (IP) Pitfalls
-
Risk of Using Counterfeit or IP-Infringing Products
Some low-cost pipette sets are direct copies of patented designs from major manufacturers (e.g., Eppendorf, Gilson, Thermo Fisher). Using such products—even unknowingly—can expose the buyer to legal risk if the original IP holder takes enforcement action, particularly in jurisdictions with strong IP protection. -
Voided Warranties and Lack of Legal Recourse
Purchasing IP-infringing equipment typically voids any manufacturer warranties. Additionally, because the supplier may operate in a legal gray area, buyers have little recourse in case of defects, safety issues, or performance failures. -
Reputational and Compliance Risk in Regulated Industries
In sectors like biotech, pharma, or clinical research, using non-genuine or IP-violating equipment may raise red flags during audits. Regulatory agencies may question data integrity if instruments lack verifiable traceability and authenticity. -
Unclear Origin and Supply Chain Transparency
Many generic pipette suppliers do not disclose manufacturing locations or design origins. This opacity increases the risk of inadvertently sourcing products tied to IP disputes or unethical manufacturing practices.
Best Practices to Mitigate Risks
- Verify Certifications: Ensure pipettes come with ISO 8655 compliance and calibration certificates traceable to national standards.
- Choose Reputable Suppliers: Source from authorized distributors or manufacturers with transparent quality control processes.
- Conduct Due Diligence on IP Status: Avoid products that too closely resemble patented designs without licensing. Request documentation on design ownership.
- Invest in Total Cost of Ownership: Consider service, calibration, and longevity—not just upfront cost.
- Include IP Clauses in Contracts: Require suppliers to warrant that products do not infringe third-party IP rights.
By proactively addressing quality and IP concerns, organizations can ensure reliable performance, regulatory compliance, and legal safety when sourcing pipette sets.

Logistics & Compliance Guide for Pipette Set
This guide outlines the essential logistics and compliance considerations for the safe, legal, and efficient handling, storage, transport, and use of a Pipette Set. Adherence to these guidelines is critical for maintaining instrument integrity, ensuring user safety, and meeting regulatory requirements.
Regulatory Compliance and Standards
Pipette Sets must comply with relevant international, national, and industry-specific regulations and standards. Key compliance areas include:
- ISO 8655: The primary international standard for piston-operated volumetric apparatus (POVA), including pipettes. It specifies performance requirements for accuracy, precision, and measurement uncertainty. Regular calibration per ISO 8655 is mandatory.
- GLP (Good Laboratory Practice): Laboratories using pipette sets for regulated testing (e.g., pharmaceuticals, environmental monitoring) must follow GLP guidelines, which include proper documentation, calibration, and traceability.
- CE Marking (EU): Pipettes sold in the European Economic Area must bear the CE mark, indicating conformity with health, safety, and environmental protection standards under EU directives.
- FDA Regulations (USA): For labs involved in clinical diagnostics or drug development, compliance with FDA 21 CFR Part 11 (electronic records) and relevant GxP standards may apply.
- RoHS and REACH (EU): Ensure pipette materials comply with restrictions on hazardous substances (RoHS) and chemical safety regulations (REACH).
Calibration and Maintenance Procedures
Regular calibration and maintenance are essential for accurate and reliable pipetting. Follow these procedures:
- Initial Calibration: Perform upon receipt and before first use. Document all baseline measurements.
- Routine Calibration: Conduct at regular intervals (e.g., every 3–6 months) or as specified by ISO 8655 and internal quality protocols.
- After Servicing or Damage: Recalibrate following any repair, accidental drop, or exposure to extreme conditions.
- Calibration Method: Use gravimetric analysis with traceable weights and controlled environmental conditions (temperature, humidity).
- Maintenance: Clean pipettes regularly with mild detergent or 70% ethanol; avoid autoclaving unless manufacturer-approved. Inspect seals and tips for wear.
Packaging and Storage Requirements
Proper packaging and storage preserve pipette functionality and prevent contamination:
- Original Packaging: Store in manufacturer-provided cases or racks to protect from dust, impact, and deformation.
- Orientation: Store vertically in designated racks to prevent liquid ingress into the body.
- Environment: Keep in a clean, dry, temperature-stable environment (15–25°C); avoid direct sunlight and high humidity.
- Contamination Control: Store away from volatile chemicals, solvents, and biological hazards to prevent material degradation or cross-contamination.
Transport and Handling Guidelines
Safe transport ensures pipettes arrive undamaged and ready for use:
- Domestic/On-Site Transport: Use padded containers or dedicated carrying cases. Secure pipettes to prevent movement.
- International/Long-Distance Shipping:
- Use shock-absorbent packaging (foam inserts, bubble wrap).
- Label packages as “Fragile” and “Laboratory Instrument.”
- Maintain chain of custody documentation for high-value or calibrated sets.
- Air Transport Compliance: Pipettes do not typically contain hazardous materials, but ensure no residual liquids remain. Follow IATA guidelines if shipping with biohazardous components.
Documentation and Traceability
Complete and accurate documentation supports compliance and quality assurance:
- Calibration Records: Maintain logs including date, technician, standard weights used, results (accuracy, precision), and next due date.
- Certificate of Conformity: Retain manufacturer-issued certificates verifying compliance with ISO 8655 and other standards.
- Usage Logs: Track usage by user, application, and any incidents (e.g., drops, malfunctions).
- Traceability: Ensure serial numbers are recorded and linked to calibration and maintenance history for audit purposes.
Safety and Environmental Considerations
Adhere to safety best practices to protect users and the environment:
- Personal Protective Equipment (PPE): Always wear gloves, lab coat, and eye protection when handling pipettes, especially with hazardous substances.
- Decontamination: Decontaminate pipettes after use with biohazardous or toxic materials following institutional biosafety protocols.
- Waste Disposal: Dispose of pipette tips and single-use components according to local biohazardous or chemical waste regulations. Recycle packaging materials where possible.
- Chemical Compatibility: Verify compatibility with solvents and reagents; prolonged exposure to aggressive chemicals (e.g., acetone, strong acids) can damage pipette seals and bodies.
Training and User Responsibility
Proper training ensures correct usage and regulatory adherence:
- User Training: All personnel must be trained in pipetting techniques, calibration awareness, and maintenance procedures.
- Standard Operating Procedures (SOPs): Implement lab-specific SOPs for pipette use, calibration, and troubleshooting.
- Accountability: Assign responsibility for calibration scheduling and record-keeping to a designated laboratory manager or quality officer.
By following this guide, organizations can ensure that their Pipette Sets remain accurate, compliant, and safe for use in regulated and research environments.
Conclusion for Sourcing a Pipette Set:
Sourcing a pipette set requires careful consideration of accuracy, precision, ergonomics, durability, and compatibility with laboratory applications. After evaluating various suppliers, brands, and product specifications, it is evident that selecting a high-quality pipette set from a reputable manufacturer ensures reliable performance, long-term cost efficiency, and compliance with industry standards. Factors such as adjustable volume range, autoclavability, ease of maintenance, and availability of calibration services further influence the decision. Ultimately, investing in a well-designed pipette set from a trusted supplier supports reproducible results, enhances laboratory productivity, and contributes to overall scientific integrity. Therefore, the recommended course of action is to procure pipettes from vetted vendors offering warranties, technical support, and adherence to quality certifications such as ISO standards.