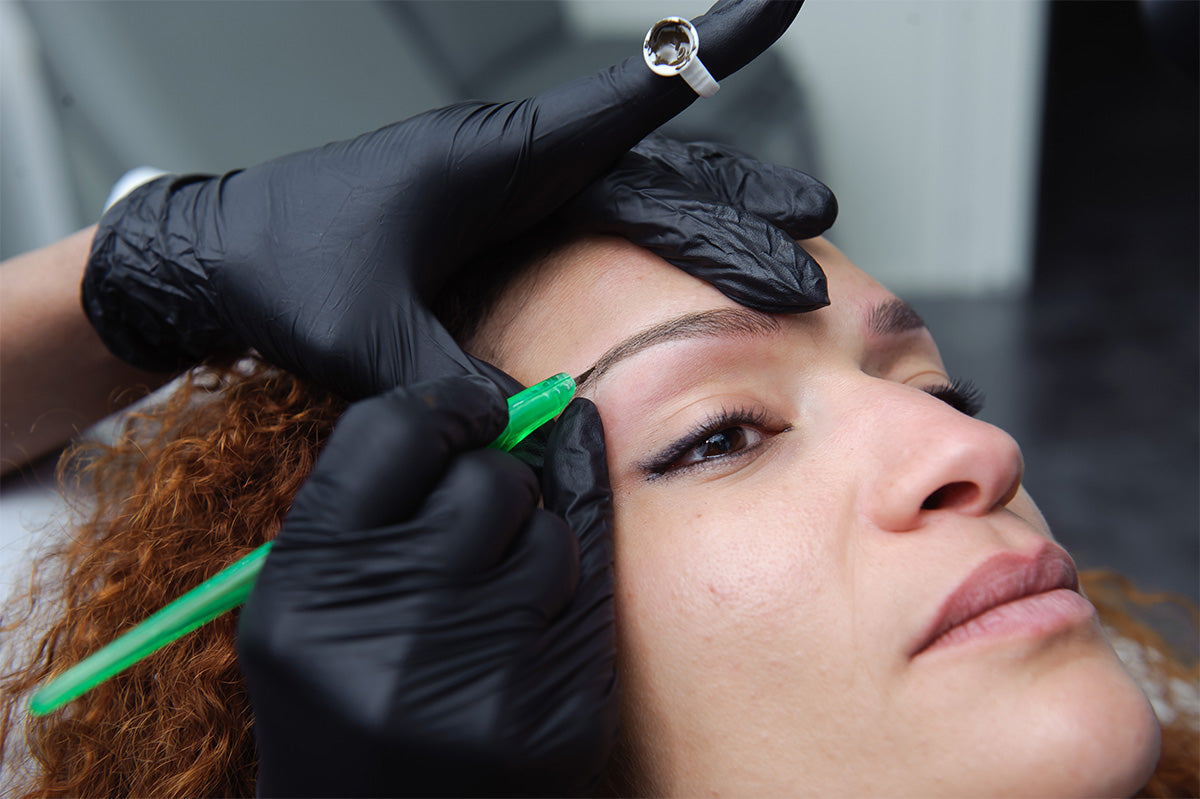
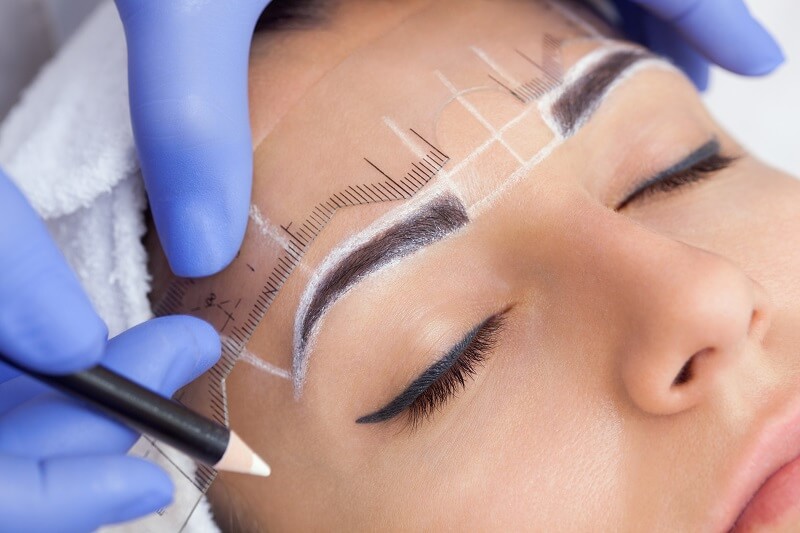
The permanent makeup (PMU) industry has experienced robust growth, fueled by increasing consumer demand for long-lasting cosmetic solutions and advancements in semi-permanent pigmentation technology. According to a report by Grand View Research, the global cosmetic tattoos market—encompassing permanent makeup—was valued at USD 2.3 billion in 2022 and is projected to expand at a compound annual growth rate (CAGR) of 7.8% from 2023 to 2030. This surge is driven by rising beauty consciousness, the growing popularity of aesthetic procedures among men and women, and the proliferation of trained PMU artists worldwide. As demand escalates, the role of reliable distributors and manufacturers becomes increasingly critical in ensuring product quality, regulatory compliance, and innovation in pigments, needles, and devices. Against this expanding backdrop, the following list highlights the top 10 permanent makeup distributors and manufacturers shaping the industry through scale, distribution reach, product performance, and market presence.
Top 10 Permanent Makeup Distributors Manufacturers 2026
(Ranked by Factory Capability & Trust Score)
#1 Li Pigments
Domain Est. 2002
Website: lipigments.com
Key Highlights: Find the perfect cosmetic pigments from over 200 pigments collection in Li Pigments. Best microblading pigments for eyes, lips, hair and makeup shades….
#2 Hanafy® Company
Domain Est. 2004
Website: hanafy.com
Key Highlights: $1 deliveryHanafy Colours Pigments is one of the largest manufacturers of pigments for permanent makeup, as well as related products necessary for performing PMU…
#3 Official Softap Distributor
Domain Est. 2017 | Founded: 1998
Website: buypermanentmakeup.com
Key Highlights: BuyPermanentMakeup.com has been an official distributor since 1998. SofTap® Permanent Cosmetics is the brand name and trademark for SofTap® Inc., ……
#4 HYVE Beauty Official
Domain Est. 2019
Website: hyvebeauty.com
Key Highlights: HYVE Beauty specializes in providing the highest quality products for a truly luxurious look. Our exclusive range of products and pigments will bring out …Missing: distributors …
#5 Permanent Makeup Products and Supplies by the International …
Domain Est. 2008
Website: permanentmakeupproducts.com
Key Highlights: $10.10 delivery 14-day returns…
#6 Perpetual Permanent Makeup Supplies Store
Domain Est. 2011
Website: perpetualpermanentmakeup.com
Key Highlights: Microblading blades, PMU machine, pen, needles, permanent makeup pigment, aftercare supplies for PMU & microblading. We ship to Canada, US & globally….
#7 Permanent Makeup Supply
Domain Est. 2016
Website: bella-tw.com
Key Highlights: Bella provides permanent makeup supply, eyelash extension, permanent make up, eyelash wave, tattoo supply and micropigmentation. The price is competitive….
#8 Biotek
Domain Est. 2017
Website: biotekmilano.com
Key Highlights: Welcome to the Official BIOTEK shop! Here you can buy our best products for permanent makeup, microblading, tattoo and microneedling….
#9 Permanent Makeup Kits
Domain Est. 2020
Website: dragonhawkofficial.com
Key Highlights: Free delivery 30-day returnsPermanent Makeup · Tattoo Needles · Tattoo Inks · Tattoo Power Supplies · Accessories. COMPANY. About Us · Contact Us · Be a Distributor · Affiliate Pro…
#10 FACE COMPANY
Domain Est. 2024
Website: facepmworld.com
Key Highlights: The shop of the manufacturer of pigments for permanent makeup using high-quality ingredients FACE COMPANY for real professionals PMU (REACH)….
Expert Sourcing Insights for Permanent Makeup Distributors
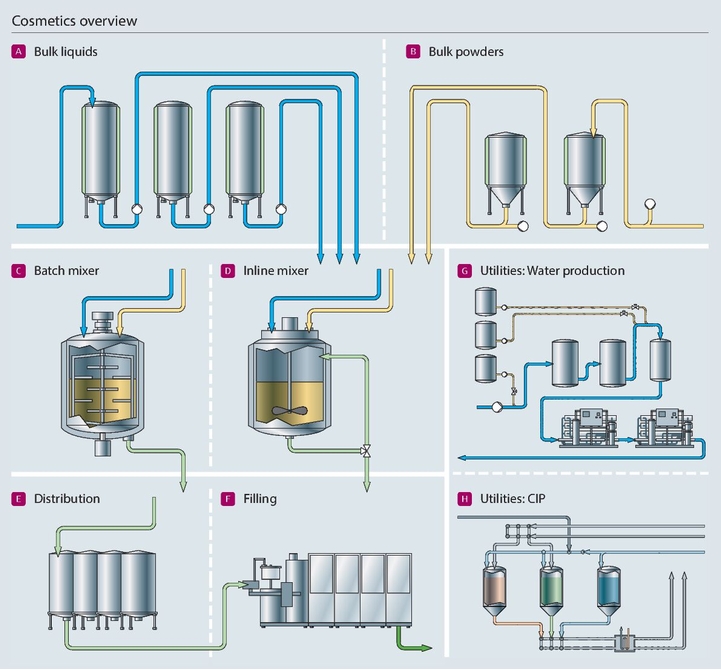
H2: 2026 Market Trends for Permanent Makeup Distributors
The permanent makeup (PMU) industry is undergoing rapid transformation, driven by technological advancements, shifting consumer preferences, and increasing demand for cosmetic enhancements. As we approach 2026, distributors in the permanent makeup sector must adapt to emerging market trends to maintain competitiveness and capitalize on growth opportunities. Below are the key trends shaping the PMU distribution landscape in 2026:
1. Rising Demand for Natural-Looking and Customized Aesthetics
Consumers are increasingly seeking subtle, personalized cosmetic enhancements that enhance facial features without appearing artificial. This shift is driving demand for high-pigment, long-lasting, and skin-tone-adaptive PMU products. Distributors are responding by expanding portfolios to include organic pigments, hypoallergenic inks, and customizable shade systems that cater to diverse skin types and undertones.
2. Growth of At-Home and Semi-Permanent Solutions
While professional PMU remains dominant, there is a growing interest in DIY and semi-permanent solutions—especially among younger demographics. In 2026, distributors are seeing increased demand for retail-friendly PMU kits, pre-drawn eyeliner stencils, and brow tinting products. Strategic partnerships with beauty retailers and e-commerce platforms are becoming essential to reach this expanding consumer base.
3. Technological Integration in PMU Devices
The integration of smart technology in PMU equipment—such as digital pigmentation machines with adjustable speed, depth control, and AI-assisted design mapping—is revolutionizing the industry. By 2026, distributors are prioritizing partnerships with manufacturers of next-gen devices that emphasize precision, safety, and ease of use. This trend is particularly strong in developed markets where practitioners demand professional-grade, efficient tools.
4. Regulatory Scrutiny and Standardization
As the PMU market grows, so does regulatory oversight. In 2026, distributors face stricter compliance requirements across key markets—including the EU’s REACH regulations and FDA guidelines in the U.S. This trend is driving a shift toward transparent sourcing, certified pigments, and comprehensive product documentation. Distributors who invest in compliance infrastructure will gain a competitive edge and build stronger trust with licensed technicians and clinics.
5. Expansion of E-Commerce and Direct-to-Consumer (DTC) Models
Digital platforms are transforming how PMU products are distributed. In 2026, B2B e-commerce portals and DTC channels are enabling faster delivery, broader reach, and data-driven inventory management. Leading distributors are leveraging AI-powered analytics to forecast demand, personalize offerings, and optimize logistics, especially in emerging markets like Southeast Asia and Latin America.
6. Sustainability and Ethical Sourcing
Environmental consciousness is influencing purchasing decisions. PMU distributors are increasingly expected to offer eco-friendly packaging, cruelty-free products, and sustainably sourced ingredients. By 2026, brands with transparent supply chains and green certifications are gaining market share, prompting distributors to reevaluate vendor partnerships and product formulations.
7. Growth in Emerging Markets
Asia-Pacific, the Middle East, and parts of Africa are witnessing a surge in PMU adoption due to rising disposable incomes and expanding beauty industries. Distributors are focusing on localized marketing, multilingual support, and region-specific training programs to support practitioners in these fast-growing regions.
Conclusion
By 2026, permanent makeup distributors must act as strategic partners in an evolving ecosystem—bridging innovation, regulation, and consumer demand. Success will depend on agility, digital integration, and a commitment to quality and sustainability. Distributors who anticipate these trends and align their offerings accordingly will be well-positioned to lead in the next era of cosmetic enhancement.

Common Pitfalls Sourcing Permanent Makeup Distributors (Quality, IP)
Sourcing permanent makeup (PMU) distributors is a critical step for brands, clinics, and entrepreneurs aiming to offer safe, effective, and legally compliant products. However, navigating this landscape comes with significant risks, particularly concerning product quality and intellectual property (IP) protection. Overlooking these pitfalls can lead to regulatory issues, customer harm, reputational damage, and legal disputes.
Poor Product Quality and Safety Standards
One of the most critical risks when sourcing PMU distributors is receiving substandard products that compromise client safety and brand integrity.
- Lack of Regulatory Compliance: Many distributors, especially overseas, may not adhere to strict cosmetic and medical device regulations such as those enforced by the FDA (U.S.), EU’s CE marking, or Health Canada. Sourcing from non-compliant suppliers can result in product recalls, fines, or import bans.
- Inconsistent Pigment Formulations: Low-quality pigments may contain harmful contaminants like heavy metals, carcinogens, or unapproved dyes. Inconsistent batch quality can lead to unpredictable results, allergic reactions, or long-term skin issues for clients.
- Sterility and Packaging Deficiencies: Permanent makeup involves breaking the skin barrier, making sterility paramount. Poorly manufactured or improperly packaged needles, cartridges, and disposables can increase the risk of infections or cross-contamination.
- Inadequate Testing and Documentation: Reputable distributors provide Certificates of Analysis (CoA), Material Safety Data Sheets (MSDS), and proof of third-party testing. Sourcing from suppliers who cannot or will not provide these documents raises red flags about product integrity.
Intellectual Property (IP) Infringement Risks
Sourcing from unethical or unscrupulous distributors can expose your business to serious intellectual property violations.
- Counterfeit or Replica Products: Some distributors sell knock-off versions of popular PMU machines, pigments, or accessories. These replicas often mimic patented designs and branded packaging, infringing on trademarks and design patents. Using or distributing such products can lead to legal action from IP holders.
- Unauthorized Use of Branding and Formulas: Distributors may falsely claim affiliation with reputable brands or use proprietary pigment names and formulations without permission. This not only dilutes brand value but can also result in cease-and-desist letters or lawsuits.
- Lack of IP Ownership Clarity: When developing private-label products, it’s crucial to ensure that the distributor isn’t using protected technology or designs. Without clear contracts defining IP ownership, your business could inadvertently infringe on patents or lose rights to custom-developed products.
- Grey Market Goods: Some distributors supply authentic products obtained through unauthorized channels. While not counterfeit, these goods may violate distribution agreements and void warranties, and selling them can breach brand protection policies.
Avoiding these pitfalls requires due diligence—vetting distributors thoroughly, demanding compliance documentation, conducting product testing, and consulting legal experts to safeguard both quality and intellectual property rights.

Logistics & Compliance Guide for Permanent Makeup Distributors
Understanding Regulatory Requirements
As a permanent makeup (PMU) distributor, compliance begins with understanding the regulatory landscape in the markets you serve. In the United States, the Food and Drug Administration (FDA) oversees the regulation of pigments and devices used in permanent makeup. While the FDA does not currently approve PMU pigments, they are subject to enforcement discretion, and distributors must ensure that all products comply with applicable laws, including proper labeling and registration. In the European Union, PMU products must comply with the EU Cosmetics Regulation (EC) No 1223/2009, which requires safety assessments, ingredient disclosure, and CE marking where applicable. Other regions, such as Canada (Health Canada) and Australia (TGA), have their own regulatory bodies and requirements. Always verify local regulations and maintain up-to-date documentation for each jurisdiction.
Product Sourcing and Supplier Verification
Distributors must establish reliable sourcing channels from manufacturers that adhere to Good Manufacturing Practices (GMP). Conduct thorough due diligence on suppliers, including audits, certifications (e.g., ISO 13485 for medical devices), and safety testing records. Ensure all pigment formulations are free from banned or restricted substances, such as certain azo dyes or heavy metals. Request Certificates of Analysis (CoA), Material Safety Data Sheets (MSDS), and allergen disclosures for every product. Maintain a supplier compliance file and require contractual agreements that enforce regulatory adherence and traceability.
Labeling and Packaging Standards
Accurate labeling is critical for compliance and consumer safety. All PMU products must be clearly labeled with the product name, ingredient list (INCI names), net quantity, batch/lot number, expiration date, manufacturer and distributor information, and usage instructions. In the EU, labels must include the responsible person and a product information file (PIF) reference. Avoid misleading claims such as “non-toxic” or “organic” unless substantiated and compliant with advertising standards. Packaging must be tamper-evident and sterile where applicable, especially for single-use cartridges and needles.
Inventory Management and Cold Chain Considerations
PMU pigments and certain devices may require specific storage conditions to maintain stability and sterility. Implement an inventory management system that tracks batch numbers, expiration dates, and storage conditions. Refrigerated storage may be necessary for certain pigment formulations—monitor and document temperature logs regularly. Use a First-Expired, First-Out (FEFO) system to prevent expired product distribution. Conduct routine audits to verify stock integrity and recall readiness.
Shipping and Distribution Logistics
Partner with carriers experienced in handling cosmetic and medical-grade goods. Ensure packaging protects contents from temperature extremes, moisture, and physical damage during transit. For international shipping, comply with customs regulations, including proper Harmonized System (HS) codes, import permits, and documentation such as commercial invoices and packing lists. Declare hazardous materials accurately (e.g., alcohol-based cleaners) and follow IATA regulations if shipping by air. Provide clear delivery instructions to retailers and maintain shipment tracking for accountability.
Post-Market Surveillance and Adverse Event Reporting
Establish a system to monitor product performance and customer feedback. Distributors are often the first point of contact for complaints or adverse events (e.g., allergic reactions, infections). Document all incidents and report serious adverse events to relevant regulatory authorities as required—such as to the FDA via MedWatch or to EU Competent Authorities via the Cosmetic Products Notification Portal (CPNP). Maintain records for a minimum of 10 years (or as mandated locally) and initiate recalls promptly if safety issues arise.
Training and Compliance Documentation
Provide comprehensive training for your team on regulatory requirements, product knowledge, and ethical distribution practices. Maintain a compliance manual that includes standard operating procedures (SOPs) for quality control, labeling, shipping, recalls, and documentation retention. Keep records of supplier agreements, product testing, certifications, and staff training to demonstrate due diligence during regulatory inspections.
Legal and Business Compliance
Ensure your business is properly registered and licensed to distribute cosmetic and medical devices in your operating regions. Obtain necessary permits, such as a Cosmetic Product Facility Registration with the FDA (in the U.S.) or registration with the CPNP (in the EU). Consult with legal counsel to review contracts, liability insurance, and intellectual property rights. Stay informed about evolving regulations, such as the EU’s upcoming revisions to cosmetic rules or U.S. state-level tattoo ink restrictions, to remain compliant and competitive.
In conclusion, sourcing permanent makeup distributors requires careful evaluation of several key factors including product quality, regulatory compliance, reputation, range of offerings, supply reliability, and customer support. Establishing partnerships with reputable distributors ensures access to safe, high-quality pigments, equipment, and aftercare products, which are essential for maintaining professional standards and client satisfaction. Additionally, choosing distributors who offer training resources and strong logistical support can significantly enhance operational efficiency for clinics and technicians. By prioritizing ethical sourcing, transparency, and long-term collaboration, businesses in the permanent makeup industry can build sustainable supply chains that support both growth and regulatory adherence in a highly specialized and competitive market.