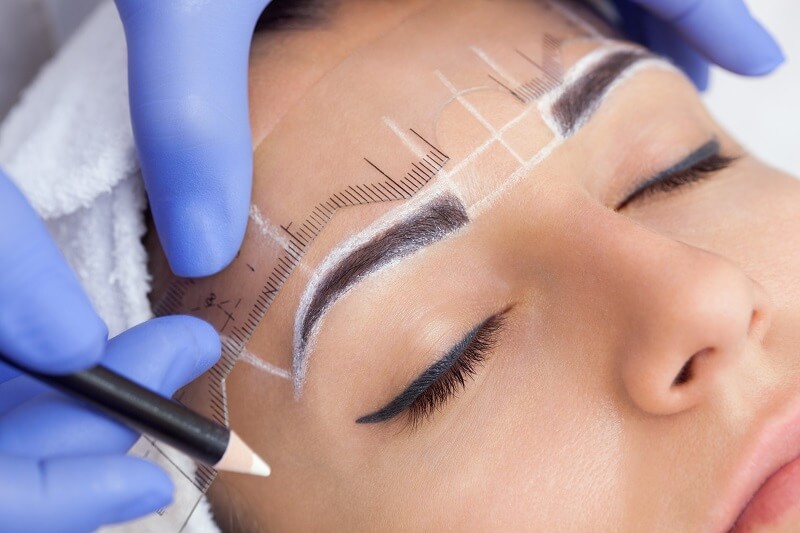
The global permanent cosmetics market is experiencing robust growth, driven by rising consumer demand for long-lasting beauty solutions and advancements in cosmetic technology. According to a report by Mordor Intelligence, the global permanent makeup market was valued at USD 6.48 billion in 2023 and is projected to reach USD 11.56 billion by 2029, growing at a CAGR of 10.2% during the forecast period. This expansion is fueled by increasing adoption of cosmetic tattooing procedures such as microblading, eyeliner tattooing, and lip pigmentation, alongside growing awareness about precision devices and hygienic practices. As demand surges, manufacturers of permanent cosmetics equipment are innovating rapidly to deliver safe, reliable, and technologically advanced tools. In this evolving landscape, a select group of manufacturers are leading the charge—setting industry standards for performance, regulatory compliance, and product ergonomics. Here, we examine the top 10 permanent cosmetics equipment manufacturers shaping the future of the beauty and aesthetics industry.
Top 10 Permanent Cosmetics Equipment Manufacturers 2026
(Ranked by Factory Capability & Trust Score)
#1 Li Pigments
Domain Est. 2002
Website: lipigments.com
Key Highlights: Find the perfect cosmetic pigments from over 200 pigments collection in Li Pigments. Best microblading pigments for eyes, lips, hair and makeup shades….
#2 Permanent Makeup Products and Supplies by the International …
Domain Est. 2008
Website: permanentmakeupproducts.com
Key Highlights: $10.10 delivery 14-day returnsGet the best deals on permanent makeup supplies, microblading, Ombre, and permanent cosmetic products with the largest selection in the Permanent Make…
#3 MLW beauty
Domain Est. 2012
Website: mlw-beauty.com
Key Highlights: MLW beauty | For over 20 years successful manufacturer of permanent make-up devices and accessories ♥ From your idea to the finished product….
#4 Permanent Makeup Kits
Domain Est. 2020
Website: dragonhawkofficial.com
Key Highlights: Free delivery 30-day returnsPermanent Makeup Kits ; Tattoo Kit | Mast Digital Tattoo Machine Professional Bundle Scalp Micro Pigmentation Device SMP · 299.00 ; Tattoo Kit | Mast P6…
#5 Perpetual Permanent Makeup Supplies Store
Domain Est. 2011
Website: perpetualpermanentmakeup.com
Key Highlights: Perpetual Permanent Makeup supplies is your premier source for microblading supplies and equipment. Our company started in this industry in 2007….
#6 Permanent Makeup Supply
Domain Est. 2016
Website: bella-tw.com
Key Highlights: Bella provides permanent makeup supply, eyelash extension, permanent make up, eyelash wave, tattoo supply and micropigmentation. The price is competitive….
#7 Biotek
Domain Est. 2017
Website: biotekmilano.com
Key Highlights: Welcome to the Official BIOTEK shop! Here you can buy our best products for permanent makeup, microblading, tattoo and microneedling….
#8 HYVE Beauty Official
Domain Est. 2019
Website: hyvebeauty.com
Key Highlights: Our exclusive range of products and pigments will bring out your unique beauty. Shop an extensive range of tools to complete the latest trends. Let us help ……
#9 PMU-line
Domain Est. 2020
Website: en.pmu-line.com
Key Highlights: PMU-line is quality products, accessories and education in the fields of permanent makeup, lash&brow treatments and mesotherapy. Visit our webshop here….
#10 Biomaser
Domain Est. 2022
Website: biomasertattoo.com
Key Highlights: Biomaser is an 20-year manufacturer of permanent makeup/microblading machine and accessories. We use high-quality products and creativity, bringing humanity ……
Expert Sourcing Insights for Permanent Cosmetics Equipment

2026 Market Trends for Permanent Cosmetics Equipment
The permanent cosmetics equipment market is poised for significant transformation by 2026, driven by technological innovation, shifting consumer preferences, and expanded global accessibility. This analysis explores key trends shaping the industry’s trajectory in the coming years.
Rising Demand for Non-Invasive Aesthetic Procedures
One of the primary drivers of growth in the permanent cosmetics equipment sector is the increasing consumer preference for minimally invasive aesthetic treatments. As individuals seek long-lasting solutions for beauty enhancement without surgery, permanent cosmetics such as microblading, eyeliner tattooing, and lip blushing are gaining popularity. According to industry forecasts, the global permanent makeup market is expected to grow at a CAGR of over 7% through 2026, directly influencing demand for advanced equipment.
Technological Advancements in Equipment Design
By 2026, permanent cosmetics equipment is expected to feature enhanced precision, safety, and ergonomics. Innovations such as digital rotary and pen-style machines with adjustable speed and needle depth settings are becoming standard. Smart devices equipped with AI-assisted pigmentation mapping and automated pigment mixing are emerging, allowing for more personalized and consistent results. Additionally, sterilization and single-use cartridge systems continue to evolve, addressing hygiene concerns and regulatory standards.
Expansion of the Global Market and Emerging Regions
While North America and Western Europe remain dominant markets, Asia-Pacific, Latin America, and the Middle East are witnessing rapid growth. Countries like India, South Korea, and Brazil are experiencing a surge in aesthetic clinics offering permanent makeup services, fueled by rising disposable incomes and growing beauty consciousness. Equipment manufacturers are responding by localizing products and offering cost-effective models tailored to regional markets.
Focus on Sustainability and Ethical Practices
Sustainability is becoming a key consideration in equipment manufacturing. By 2026, brands are expected to prioritize eco-friendly materials, energy-efficient devices, and recyclable packaging. Additionally, there is growing demand for cruelty-free and vegan-certified equipment and pigments, aligning with broader consumer values around ethical beauty.
Regulatory Harmonization and Safety Standards
As the industry matures, regulatory bodies worldwide are tightening standards for permanent cosmetics equipment. The U.S. FDA, EU’s CE marking, and other regional authorities are enforcing stricter guidelines on device safety, electromagnetic compatibility, and biocompatibility. Manufacturers are investing in compliance and certification to ensure global market access, which is expected to improve consumer trust and reduce complications.
Integration with Digital Platforms and Training Tools
Digital transformation is extending into training and practice. By 2026, virtual reality (VR) and augmented reality (AR) platforms are anticipated to be widely used for technician training, allowing for simulated practice and real-time feedback. Online marketplaces and certification programs will further professionalize the field, increasing demand for high-quality, compatible equipment.
Conclusion
The 2026 landscape for permanent cosmetics equipment will be defined by innovation, globalization, and heightened consumer expectations. As the market evolves, stakeholders—from manufacturers to practitioners—must adapt to technological advances, regulatory changes, and sustainability imperatives to remain competitive and deliver safe, effective, and ethical solutions.

Common Pitfalls When Sourcing Permanent Cosmetics Equipment: Quality and Intellectual Property Risks
Sourcing permanent cosmetics equipment—such as tattoo and micropigmentation machines, power supplies, and accessories—can be challenging, especially when balancing cost, quality, and legal compliance. Two of the most critical areas where businesses encounter problems are equipment quality and intellectual property (IP) infringement. Overlooking these aspects can lead to safety issues, regulatory penalties, brand damage, and legal disputes.
Poor Equipment Quality and Safety Standards
One of the most frequent pitfalls is prioritizing low cost over quality, resulting in equipment that is unreliable, unsafe, or non-compliant with industry regulations.
- Substandard Materials and Construction: Cheaply manufactured devices may use inferior motors, plastics, or electrical components that wear out quickly or malfunction during procedures, increasing the risk of injury or inconsistent pigment deposition.
- Lack of Medical-Grade Certification: In many regions, permanent cosmetics equipment must meet medical device standards (such as ISO 13485 or CE marking for medical devices). Sourcing non-certified equipment exposes practitioners to legal liability and may void insurance coverage.
- Inconsistent Performance: Low-quality machines often deliver inconsistent needle speed or depth, leading to poor aesthetic outcomes and client dissatisfaction.
- Inadequate Sterilization Compatibility: Some equipment cannot withstand standard autoclaving processes, increasing the risk of cross-contamination and failing health and safety audits.
To mitigate these risks, always verify certifications, request product testing reports, and source from reputable manufacturers with a track record in the aesthetic or medical device industry.
Intellectual Property (IP) Infringement
Another significant but often overlooked risk is purchasing equipment that infringes on third-party patents, trademarks, or design rights.
- Counterfeit or Clone Devices: Many suppliers, particularly on online marketplaces, offer devices that closely mimic patented technology from leading brands. These clones may appear identical but are produced without licensing, exposing buyers to legal risk.
- Use of Branded Components or Logos: Equipment that features unauthorized use of well-known brand names or logos can lead to trademark infringement claims, resulting in seized shipments or cease-and-desist orders.
- Patent Violations in Technology Design: Advanced features such as wireless control, digital calibration, or ergonomic designs may be protected by patents. Using or importing infringing devices—even unknowingly—can result in costly litigation.
- Limited Recourse from Unethical Suppliers: If IP disputes arise, generic suppliers often lack the legal infrastructure to defend or indemnify buyers, leaving the end user vulnerable.
To protect your business, conduct due diligence on suppliers, request proof of IP ownership or licensing, and avoid deals that seem too good to be true. Working with original equipment manufacturers (OEMs) or authorized distributors significantly reduces IP risks.
By focusing on certified quality and respecting intellectual property rights, businesses can ensure safer practices, maintain compliance, and build a trustworthy reputation in the permanent cosmetics industry.

Logistics & Compliance Guide for Permanent Cosmetics Equipment
Overview and Purpose
This guide provides essential information for the safe, legal, and efficient handling, transportation, and use of permanent cosmetics equipment. It ensures compliance with international, national, and industry-specific regulations related to medical devices, cosmetics, and health and safety standards.
Regulatory Classification
Permanent cosmetics equipment—such as tattoo machines, micropigmentation devices, power supplies, and needle cartridges—is typically classified as a medical device or cosmetic tool depending on jurisdiction. In the United States, the FDA regulates these devices under 21 CFR Part 878 (General and Plastic Surgery Devices). In the European Union, compliance with the Medical Devices Regulation (MDR) (EU) 2017/745 may apply if the equipment is intended for dermal implantation or permanent modification.
Import and Export Requirements
- Customs Documentation: Ensure accurate Harmonized System (HS) codes are used (e.g., 8543.70 for electrical therapeutic apparatus).
- Country-Specific Regulations: Verify import requirements in destination countries, including conformity assessments, labeling, and registration.
- Restricted Substances: Confirm equipment complies with REACH (EU), RoHS, and other chemical safety directives.
Shipping and Handling Procedures
- Packaging: Use tamper-evident, sterile, and shock-resistant packaging to protect sensitive components.
- Temperature Control: Avoid exposure to extreme temperatures during transit; store and ship between 15°C and 25°C (59°F–77°F).
- Labeling: Clearly label packages with handling instructions (e.g., “Fragile,” “Do Not Freeze,” “Sterile Equipment”).
Sterility and Infection Control Compliance
- Single-Use Components: Ensure needles, cartridges, and tubing are individually packaged and labeled as sterile and for single use only.
- Reusable Equipment: Follow manufacturer-recommended sterilization protocols (e.g., autoclaving at 121°C for 15–20 minutes, per ISO 17664).
- Validation: Maintain documentation for sterilization cycles and equipment cleaning logs where applicable.
Labeling and User Instructions
- Multilingual Labels: Include warnings, intended use, and contraindications in the local language(s) of the destination market.
- Instructions for Use (IFU): Provide comprehensive IFUs covering setup, operation, maintenance, and disposal.
- CE Marking or FDA Listing: Display required certification marks based on market approval.
Training and Certification for End Users
- Certified Training: Operators must complete accredited permanent cosmetics training programs covering hygiene, anatomy, and emergency procedures.
- Compliance with Local Laws: Verify state or national licensing requirements (e.g., health department permits in the U.S.).
Waste Disposal and Environmental Compliance
- Sharps Disposal: Used needles and cartridges must be discarded in FDA-cleared sharps containers and disposed of per biohazard waste regulations (e.g., OSHA Bloodborne Pathogens Standard).
- Electronic Waste (E-Waste): Recycle power units and electronic components through certified e-waste programs compliant with WEEE (EU) or local regulations.
Recordkeeping and Audit Readiness
- Traceability: Maintain batch records, serial numbers, and distribution logs for recall readiness.
- Compliance Documentation: Keep copies of regulatory approvals, sterilization certificates, training records, and safety data sheets (SDS) for at least 5 years.
Incident Reporting and Recalls
- Adverse Event Reporting: Report malfunctions or injuries to relevant authorities (e.g., FDA MedWatch, EU Vigilance System) within required timeframes.
- Recall Procedures: Establish a recall plan including notification protocols, return logistics, and root cause analysis.
Conclusion
Adhering to logistics and compliance standards ensures the safety of clients, protects practitioners from liability, and supports market access. Regularly review evolving regulations and maintain close coordination with legal, regulatory, and supply chain partners.
Conclusion for Sourcing Permanent Cosmetics Equipment
Sourcing permanent cosmetics equipment is a critical step in establishing or expanding a professional and safe practice in the field of cosmetic tattooing. The process requires careful consideration of quality, safety, compliance, and long-term reliability. Choosing FDA-compliant or internationally certified devices from reputable manufacturers ensures not only the safety and satisfaction of clients but also compliance with health and regulatory standards.
Key factors such as precision, ease of use, after-sales support, warranty, and sterilization compatibility should guide the selection process. Additionally, investing in comprehensive training and ongoing education when adopting new equipment enhances treatment outcomes and builds client trust. While cost is a consideration, prioritizing quality over short-term savings helps prevent equipment failure, reduce downtime, and maintain professional credibility.
Ultimately, sourcing the right permanent cosmetics equipment is an investment in both the practitioner’s expertise and the client’s experience. By partnering with trusted suppliers and staying informed about technological advancements, professionals can ensure efficient, hygienic, and effective treatments, positioning themselves for long-term success in this growing industry.