The global PCR (Polymerase Chain Reaction) machine market has experienced robust growth, driven by rising demand for molecular diagnostics, increased investment in life sciences research, and expanding applications in clinical, academic, and biopharmaceutical sectors. According to a report by Mordor Intelligence, the PCR market was valued at USD 6.74 billion in 2023 and is projected to grow at a CAGR of 7.8% from 2024 to 2029. Similarly, Grand View Research estimated the market at USD 6.43 billion in 2022, with a projected CAGR of 7.6% over the same forecast horizon. This sustained expansion reflects increasing adoption of advanced PCR technologies—including real-time qPCR and digital PCR—amid growing infectious disease burdens, personalized medicine initiatives, and genomic research. As demand intensifies, several manufacturers have emerged as key innovators, combining precision engineering, automation, and scalable solutions to meet diverse laboratory needs. Here are the top 9 PCR machine manufacturers shaping the future of molecular analysis.
Top 9 Pcr Machien Manufacturers 2026
(Ranked by Factory Capability & Trust Score)
#1 Next Generation PCR
Domain Est. 2002
Website: isogen-lifescience.com
Key Highlights: The NextGenPCR machines use innovative and patented heating technology to perform ultrafast amplification of nucleic acid sequences from biological materials….
#2 PCRmax PCR / qPCR Thermal Cyclers and Products
Domain Est. 1994
Website: coleparmer.com
Key Highlights: Same great products and quality, new name! Ensure quality results! PCRmax thermal cyclers for PCR and qPCR offer outstanding temperature stability….
#3 PCR Instruments
Domain Est. 1997
Website: qiagen.com
Key Highlights: Our real-time and end-point PCR cyclers and digital PCR instruments are easy to use and deliver accurate, sensitive results….
#4 ProFlex PCR Systems
Domain Est. 2006
Website: thermofisher.com
Key Highlights: Learn more about ProFlex PCR Systems/ProFlex thermal cyclers: flexible, high-throughput PCR systems with multiple blocks to run separate experiments….
#5 Nio®: 7-color digital PCR solution
Domain Est. 2012
Website: stillatechnologies.com
Key Highlights: Nio Digital PCR offers a simple digital PCR workflow that supports 7 colors, a capacity of up to 768 samples per 8-hour workday, continuous loading and, easy- ……
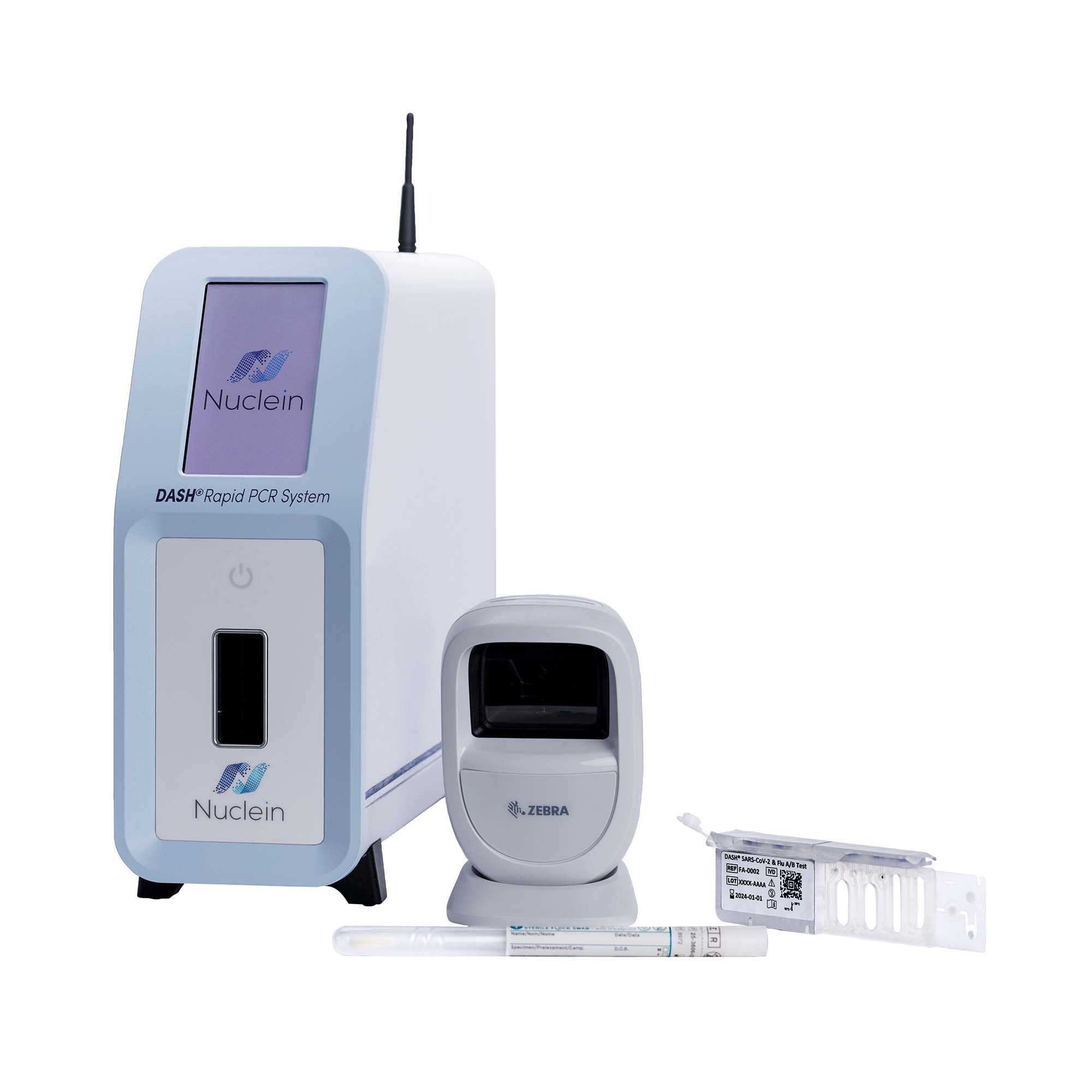
#6 Nuclein DASH Rapid PCR System
Domain Est. 2016
Website: nuclein.com
Key Highlights: DASH™ Rapid PCR System is a simple, affordable, point-of-care platform that performs sample-to-answer PCR in 15 minutes….
#7 Automated PCR Machines
Domain Est. 2016
Website: analytik-jena.us
Key Highlights: Automated PCR systems enable higher throughput while achieving excellent results. Shop our collection of automation-friendly PCR and RT-PCR machines today!…
#8 3CR Bioscience
Domain Est. 2017
Website: 3crbio.com
Key Highlights: A leading provider of innovative PCR genotyping products and services for agriculture and life sciences – Making science affordable….
#9 Co
Domain Est. 2021
Website: co-dx.com
Key Highlights: The Co-Dx PCR platform gives your organization rapid real-time test results, so you can make better decisions to keep everyone safe….
Expert Sourcing Insights for Pcr Machien

H2: Market Trends for PCR Machines in 2026
As the global healthcare and life sciences sectors continue to evolve, the Polymerase Chain Reaction (PCR) machine market is poised for significant transformation by 2026. Driven by technological innovation, increasing demand for point-of-care diagnostics, and expanding applications beyond traditional infectious disease testing, the PCR machine landscape is entering a phase of accelerated growth and diversification. Below are the key market trends expected to shape the PCR machine industry in 2026.
1. Shift Toward Point-of-Care and Portable PCR Systems
One of the most prominent trends in 2026 is the growing adoption of compact, portable, and rapid PCR devices. These point-of-care (POC) systems enable near-patient testing in clinical settings, remote areas, and even home environments. Advances in microfluidics and miniaturization technologies have made handheld and benchtop PCR machines more reliable and user-friendly. This shift is particularly driven by the need for rapid diagnostics in low-resource settings and decentralized healthcare models.
2. Integration of Automation and AI
Automation is becoming a standard feature in next-generation PCR machines. By 2026, many systems will incorporate robotic sample handling, automated data analysis, and artificial intelligence (AI)-driven interpretation of results. This integration reduces human error, increases throughput, and allows integration with laboratory information management systems (LIMS), enhancing overall workflow efficiency in clinical and research labs.
3. Expansion in Non-Infectious Disease Applications
While PCR has long been a cornerstone of infectious disease diagnostics, its use is expanding into oncology, genetic testing, and personalized medicine. In 2026, PCR machines are increasingly used for liquid biopsy analysis, cancer mutation detection, and pharmacogenomics. This diversification is fueling demand in hospitals, specialized diagnostic centers, and biopharmaceutical R&D facilities.
4. Growth in Emerging Markets
Asia-Pacific, Latin America, and parts of Africa are expected to register the highest growth rates for PCR machine adoption by 2026. Government investments in public health infrastructure, rising awareness of molecular diagnostics, and increasing prevalence of infectious and chronic diseases are key drivers. Local manufacturing and partnerships with global players are also reducing costs and improving market access.
5. Emphasis on Sustainability and Cost-Efficiency
With growing environmental concerns, manufacturers are focusing on energy-efficient PCR machines and recyclable consumables. In 2026, eco-conscious design and reduced reagent waste are becoming competitive differentiators. Additionally, open-platform systems that allow third-party reagents are gaining traction, reducing operational costs for end users.
6. Consolidation and Competitive Landscape Evolution
The PCR machine market is witnessing increased consolidation, with major players acquiring innovative startups to enhance their product portfolios. Companies like Thermo Fisher Scientific, Roche, Bio-Rad, and Qiagen are investing heavily in R&D to launch integrated, high-throughput systems. At the same time, new entrants are focusing on niche applications and AI-powered diagnostics, intensifying competition.
7. Regulatory Harmonization and Standardization
By 2026, regulatory bodies worldwide are moving toward harmonized standards for molecular diagnostic devices, including PCR machines. This trend simplifies market entry for manufacturers and ensures greater reliability and interoperability of diagnostic results across regions, particularly important in global health initiatives and pandemic preparedness.
Conclusion
The PCR machine market in 2026 is characterized by technological sophistication, broader application scope, and increasing accessibility. As healthcare systems prioritize precision, speed, and decentralization, PCR technology remains at the forefront of molecular diagnostics. Stakeholders who embrace innovation, expand into emerging applications, and adapt to evolving regulatory and environmental demands will be best positioned to succeed in this dynamic landscape.

Common Pitfalls When Sourcing PCR Machines: Quality and Intellectual Property Risks
Poor Quality and Performance Issues
One of the most frequent challenges when sourcing PCR machines—especially from lesser-known or overseas suppliers—is receiving equipment that does not meet expected performance standards. Low-cost machines may use substandard components, leading to inconsistent temperature control, poor thermal uniformity across wells, and unreliable amplification results. These issues can compromise experimental accuracy, increase reagent waste, and ultimately delay research or diagnostic workflows. Additionally, inadequate customer support and limited service networks make troubleshooting and repairs difficult, further reducing machine uptime and reliability.
Intellectual Property Infringement Risks
Sourcing PCR machines from certain manufacturers, particularly those in regions with weak IP enforcement, raises concerns about potential intellectual property violations. Some machines may incorporate patented thermal cycling technologies, software algorithms, or design elements without proper licensing. Purchasing such equipment—even unknowingly—can expose institutions or companies to legal liability, especially in regulated environments like clinical diagnostics or commercial R&D. Furthermore, using non-compliant devices may void warranties or prevent integration with proprietary reagent systems, limiting functionality and scalability. Conducting due diligence on manufacturer legitimacy and IP compliance is essential to mitigate these risks.
Logistics & Compliance Guide for PCR Machines
PCR (Polymerase Chain Reaction) machines are sensitive diagnostic instruments used widely in clinical, research, and public health settings. Proper logistics and compliance management are essential to ensure functionality, regulatory approval, and patient safety. This guide outlines key considerations for transporting, handling, and maintaining regulatory compliance for PCR machines.
Regulatory Classification and Documentation
PCR machines are typically classified as in vitro diagnostic (IVD) medical devices under international regulatory frameworks. In the U.S., they are regulated by the FDA under 21 CFR Part 866, while in the EU, they fall under the In Vitro Diagnostic Regulation (IVDR) (EU) 2017/746. Ensure the device has the appropriate regulatory certification (e.g., FDA 510(k), CE marking, or local approvals) before shipment. Maintain copies of technical files, Declaration of Conformity, labeling, and user manuals for customs and inspection purposes.
Packaging and Transportation
Use manufacturer-recommended packaging with shock-absorbing materials to protect the instrument from vibrations, drops, and temperature fluctuations. Include desiccants if required to control moisture. Ship in original packaging whenever possible. Clearly label packages with “Fragile,” “This Side Up,” and “Protect from Moisture.” Use temperature-controlled transport if the device is sensitive to extreme heat or cold (typically -10°C to 50°C for storage, 15°C–30°C for operation). Consider using GPS and environmental monitoring devices during transit to track location, temperature, and shock events.
Import/Export Compliance
Verify export controls under your country’s regulations (e.g., U.S. Commerce Control List or EU Dual-Use Regulation). PCR machines may require export licenses depending on destination countries. Prepare accurate commercial invoices, packing lists, and a certified statement of non-clinical use if applicable. Classify the device correctly under the Harmonized System (HS) Code—typically under 9018.19 or 9027.80 for diagnostic equipment. Confirm import requirements in the destination country, including registration with local health authorities (e.g., ANVISA in Brazil, NMPA in China).
Installation and Site Readiness
Ensure the installation site meets minimum environmental conditions: stable power supply (with surge protection), controlled ambient temperature (18–25°C), low humidity (30–70%), and minimal dust. Provide a level, vibration-free surface. Verify electrical compatibility (voltage, frequency, grounding). Install in a biosafety-compliant area if used for clinical testing. Coordinate with technical support for on-site calibration and qualification (IQ/OQ) upon delivery.
Maintenance and Service Compliance
Follow the manufacturer’s recommended maintenance schedule and keep detailed service records. Only authorized personnel should perform repairs or modifications. Ensure software updates are validated and compliant with regulatory standards (e.g., FDA 21 CFR Part 11 for electronic records). Maintain audit trails if the device interfaces with Laboratory Information Systems (LIS).
Data Security and Privacy
If the PCR machine stores or transmits patient data, ensure compliance with data protection laws such as GDPR, HIPAA, or local privacy regulations. Implement access controls, encryption, and data backup protocols. Confirm that data handling complies with institutional or national biosafety and bioethics policies.
Disposal and End-of-Life Management
Dispose of obsolete or damaged PCR machines in accordance with local e-waste and medical device disposal regulations. Remove all data storage components and securely erase any stored information. Coordinate with certified e-waste recyclers or the manufacturer’s take-back program to ensure environmentally responsible disposal.
By adhering to these logistics and compliance guidelines, organizations can ensure the safe, legal, and effective deployment of PCR machines across global operations.
Conclusion for Sourcing a PCR Machine:
After a thorough evaluation of technical specifications, supplier credibility, cost-effectiveness, after-sales support, and user requirements, sourcing a PCR machine requires a balanced approach that prioritizes reliability, accuracy, and long-term operational efficiency. It is essential to select a machine that aligns with the laboratory’s throughput needs, application scope (e.g., qualitative, quantitative, or real-time PCR), and future scalability. Engaging with reputable suppliers, conducting due diligence on warranty and service agreements, and considering total cost of ownership—rather than upfront price alone—will ensure a successful investment. Ultimately, the chosen PCR machine should enhance diagnostic or research capabilities, support consistent results, and integrate smoothly into existing workflows, contributing to overall scientific and operational success.