The global micropipette market continues to expand, driven by increasing demand in life sciences, pharmaceuticals, and academic research. According to Mordor Intelligence, the micropipette market was valued at approximately USD 1.3 billion in 2023 and is projected to grow at a CAGR of over 6.5% through 2029. This growth is fueled by rising investments in R&D, advancements in liquid handling technologies, and the expansion of biotechnology applications worldwide. As laboratories seek precision, ergonomics, and compatibility with automation, the demand for high-quality, affordable micropipettes—particularly in the sub-P200 range—has intensified. This surge has led to increased competition among manufacturers to deliver reliable, accurate, and cost-effective solutions. Based on market presence, innovation, and user reviews, the following five companies stand out as leading manufacturers of P200 micropipettes, offering products that balance performance and value for diverse laboratory workflows.
Top 5 P200 Micropipette Manufacturers 2026
(Ranked by Factory Capability & Trust Score)
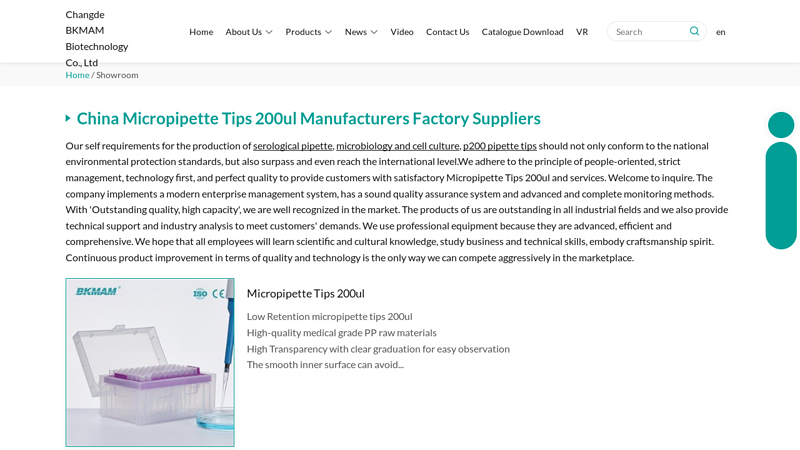
#1 China Micropipette Tips 200ul Manufacturers Factory
Domain Est. 2022
Website: bkmbio.com
Key Highlights: BKMAM is one of the most professional pipette tips manufacturers in China, specialized in providing high quality products and service….
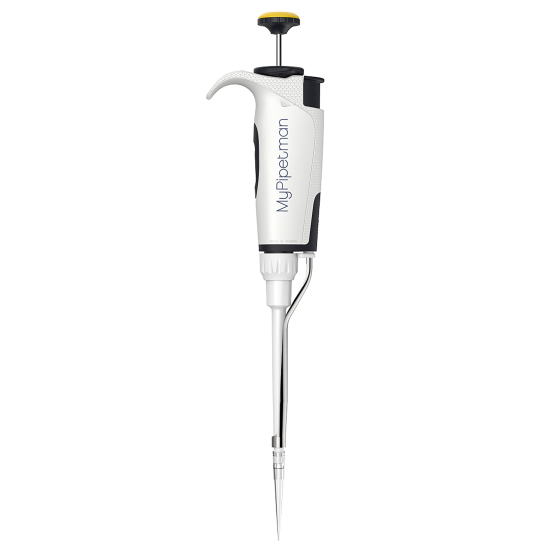
#2 MyPIPETMAN Select P200, 20
Domain Est. 1995
Website: gilson.com
Key Highlights: In stock Free deliveryErgonomic, safe, and reliable, MyPIPETMAN® Select offers volume setting choice and optional handle personalization for optimal use in various protocols.Missin…
#3 Edvotek® Variable Micropipette 20
Domain Est. 1997
Website: edvotek.com
Key Highlights: Highly-accurate, single channel manual p200 micropipette. $99. Uses standard pipette tips. Lifetime Warranty!…
#4 P200
Domain Est. 1998
Website: sigmaaldrich.com
Key Highlights: Find p200-pipette and related products for scientific research at MilliporeSigma….
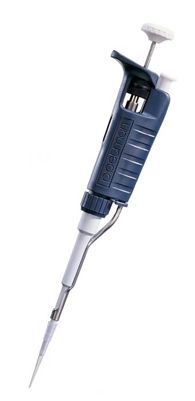
#5 Gilson PIPETMAN Classic P200 Pipette
Domain Est. 2012
Website: new.marshallscientific.com
Key Highlights: Out of stockThe Gilson PIPETMAN Classic P200 Pipette is discounted, brand-new, and comes with a standard full parts and labor warranty….
Expert Sourcing Insights for P200 Micropipette

H2: Projected 2026 Market Trends for P200 Micropipette
The global market for P200 micropipettes—precision instruments used for measuring and transferring liquid volumes between 20–200 µL—is anticipated to experience significant evolution by 2026, driven by advancements in laboratory automation, rising demand in life sciences research, and increased emphasis on accuracy and reproducibility in scientific workflows. Several key trends are expected to shape the P200 micropipette market in the coming years:
-
Growth in Life Sciences and Biopharmaceutical R&D
By 2026, continued expansion in genomics, proteomics, and drug discovery will sustain strong demand for high-precision micropipettes like the P200. Biopharmaceutical companies and academic research institutions are investing heavily in high-throughput screening and molecular biology techniques—applications that rely heavily on reliable liquid handling. The P200, being one of the most commonly used pipette volumes, will remain a staple in labs worldwide. -
Shift Toward Electronic and Connected Micropipettes
A major trend shaping the 2026 market is the growing adoption of electronic P200 micropipettes equipped with digital displays, programmable protocols, and data logging capabilities. Integration with Laboratory Information Management Systems (LIMS) and cloud-based platforms enhances traceability and compliance, especially in regulated environments such as clinical diagnostics and GxP labs. Major manufacturers like Eppendorf, Thermo Fisher Scientific, and Mettler Toledo are expected to expand their portfolios of smart pipetting solutions. -
Emphasis on Ergonomics and User Safety
Repetitive strain injuries (RSIs) associated with manual pipetting are driving innovation in ergonomic design. By 2026, manufacturers will increasingly offer lightweight, balanced P200 models with reduced plunger forces and advanced tip ejection mechanisms. These features are not only improving user comfort but also reducing variability caused by operator fatigue—critical for data integrity. -
Sustainability and Green Laboratory Initiatives
Environmental concerns are influencing product design and procurement decisions. The market will see a rise in recyclable pipette tips, refillable calibration systems, and longer-lasting battery-powered electronic pipettes. Laboratories aiming for sustainability certifications (e.g., LEED, My Green Lab) will favor P200 models with lower environmental footprints. -
Regional Market Expansion
While North America and Europe will remain dominant markets due to mature research infrastructures, Asia-Pacific—particularly China, India, and South Korea—is expected to experience the highest growth rate by 2026. Government funding for biotechnology and expanding CRO/CDMO sectors are accelerating lab equipment demand, including P200 micropipettes. Local manufacturing and cost-effective alternatives will gain traction in emerging economies. -
Impact of Automation and Integration
As automated liquid handling systems become more accessible, standalone P200 pipettes will increasingly be designed to interface with robotic platforms. Modular designs and compatibility with multichannel configurations will enhance versatility, allowing seamless integration into semi-automated workflows. -
Price Competition and Aftermarket Services
The market is expected to witness intensified competition between premium brands and emerging manufacturers offering high-quality, lower-cost alternatives. Additionally, revenue from calibration, repair, and tip supply services will grow, with vendors offering comprehensive service packages to strengthen customer retention.
In conclusion, the 2026 market for P200 micropipettes will be characterized by innovation in smart technology, ergonomic design, and sustainability, underpinned by strong demand from the life sciences sector. Vendors that prioritize precision, user experience, and digital integration are likely to lead the market, while cost-effective solutions will capture growing shares in developing regions.

Common Pitfalls When Sourcing a P200 Micropipette: Quality and Intellectual Property Risks
When sourcing a P200 micropipette, organizations often encounter significant challenges related to product quality and intellectual property (IP) infringement. Overlooking these pitfalls can lead to compromised experimental accuracy, equipment failure, legal exposure, and reputational damage. Understanding these risks is crucial for making informed procurement decisions.
Quality-Related Pitfalls
Inconsistent Calibration and Accuracy
One of the most prevalent quality issues with low-cost or uncertified P200 micropipettes is inconsistent volumetric accuracy. Many off-brand pipettes fail to meet ISO 8655 standards, leading to volume dispersions outside acceptable tolerances. This variability directly impacts reproducibility in sensitive applications like PCR, ELISA, or serial dilutions, potentially invalidating research results.
Poor Build Quality and Durability
Counterfeit or substandard micropipettes often use inferior materials, such as low-grade plastics and weak internal springs. This results in reduced longevity, inconsistent plunger action, and increased susceptibility to damage from routine use or decontamination procedures. Users may experience tip ejection failure, leakage, or complete mechanical breakdown after minimal use.
Lack of Traceable Calibration and Certification
Reputable manufacturers provide individual calibration certificates with NIST-traceable documentation. However, many non-OEM (original equipment manufacturer) or gray-market pipettes lack verifiable certification. Without proper documentation, laboratories may fail to meet regulatory compliance requirements (e.g., GLP, ISO 17025), risking audit findings or data rejection.
Intellectual Property (IP) Risks
Counterfeit and Clone Products
The market is flooded with micropipettes that mimic the design and branding of established manufacturers like Eppendorf, Gilson, or Thermo Fisher. These counterfeit products often infringe on design patents, trademarks, and utility patents. Purchasing such items exposes the buyer to potential IP liability, especially in commercial or regulated environments.
Voided Warranties and Lack of Support
Using non-genuine or cloned pipettes typically voids manufacturer warranties and denies access to authorized service, recalibration, and technical support. This not only increases long-term costs due to unavailability of spare parts but also disrupts laboratory workflows when repairs are needed.
Ethical and Institutional Compliance Concerns
Many academic and research institutions have procurement policies that prohibit the use of counterfeit or IP-infringing equipment. Sourcing cloned pipettes may violate these policies, leading to internal compliance issues or jeopardizing grant funding that requires adherence to ethical sourcing standards.
Conclusion
To mitigate these risks, laboratories should procure P200 micropipettes only through authorized distributors, verify product authenticity, and prioritize suppliers that provide full documentation, including calibration certificates and compliance statements. Investing in genuine, high-quality equipment ensures data integrity, regulatory compliance, and long-term cost efficiency, while safeguarding against legal and ethical pitfalls.

H2: Logistics & Compliance Guide for P200 Micropipette
H2.1: Shipping & Handling
- Packaging: Ensure the P200 micropipette is securely stored in its original protective case or padded container to prevent mechanical damage during transit.
- Labeling: Clearly label packages containing micropipettes as “Fragile – Laboratory Equipment” and “Do Not Invert” to maintain piston seal integrity.
- Storage Conditions: Store at temperatures between 5°C and 40°C, in a dry, dust-free environment away from direct sunlight and chemical vapors.
- Transport: Avoid exposure to extreme temperatures or humidity during shipping. Use climate-controlled transport for international or long-distance deliveries.
H2.2: Regulatory Compliance
- CE Marking: Confirm the P200 micropipette complies with EU directives (e.g., RoHS, WEEE) and bears the CE mark when distributed in Europe.
- ISO Standards: Ensure the device meets relevant standards, including ISO 8655 (requirements for piston-operated volumetric apparatus) and ISO 9001 (quality management).
- US FDA/NIH Guidelines: For clinical or diagnostic use in the US, verify compliance with FDA 21 CFR Part 820 (Quality System Regulation) and NIH best practices for laboratory instrumentation.
- Country-Specific Regulations: Check local requirements (e.g., Health Canada, TGA in Australia, ANVISA in Brazil) before import and commercial distribution.
H2.3: Calibration & Traceability
- Initial Calibration: Perform calibration upon receipt using traceable standards (NIST or equivalent) to verify volume accuracy and precision within ±2% for the P200 range (20–200 µL).
- Schedule: Recalibrate every 6–12 months, or more frequently under heavy use or after impact.
- Documentation: Maintain calibration records including date, technician, standard used, results, and next due date. Store digitally with backup.
- Traceability: Use certified reference liquids (e.g., distilled water at specified temperature) and calibrated balances for gravimetric testing.
H2.4: User Training & Documentation
- Training: Provide documented training for all users on proper operation, handling, and maintenance to prevent damage and ensure accuracy.
- User Manual: Retain the manufacturer’s instruction manual on-site and ensure digital access. Include details on volume adjustment, tip ejection, and error codes.
- SOPs: Implement Standard Operating Procedures (SOPs) for pipetting technique, daily checks, and pre-use inspection.
H2.5: Maintenance & Repair
- Cleaning: Decontaminate regularly using 60–70% ethanol or manufacturer-approved solutions. Avoid immersing the body or using autoclaving unless specified.
- Autoclaving: If applicable, disassemble as instructed and autoclave at 121°C for 20 minutes with slow exhaust. Allow to cool completely before reassembly.
- Servicing: Use only authorized service centers for repairs to maintain warranty and compliance. Keep service logs for audits.
- Part Replacement: Replace worn seals, O-rings, or tip cones according to schedule or visible wear.
H2.6: Environmental & Disposal Compliance
- End-of-Life Disposal: Follow WEEE (Waste Electrical and Electronic Equipment) guidelines in applicable regions. Do not dispose of as regular trash.
- Battery Handling (if applicable): Remove and recycle batteries separately per local hazardous waste regulations.
- Recycling: Contact the manufacturer or certified e-waste handler for responsible recycling of electronic components and plastics.
H2.7: Audit & Recordkeeping
- Inspection Logs: Maintain records of routine inspections, usage, and issues.
- Audit Readiness: Ensure all documentation (calibration, training, maintenance) is organized and accessible for internal or regulatory audits.
- Data Integrity: Where electronic records are used, follow ALCOA+ principles (Attributable, Legible, Contemporaneous, Original, Accurate, plus Complete, Consistent, Enduring, Available).
Note: Always refer to the manufacturer’s specific guidelines for the P200 micropipette model in use, as specifications and compliance requirements may vary.
Conclusion for Sourcing P200 Micropipette:
After evaluating various suppliers, brands, and cost considerations, sourcing a P200 micropipette requires a balance between accuracy, durability, ergonomics, and long-term value. Reputable manufacturers such as Eppendorf, Gilson, and Thermo Fisher Scientific offer reliable and well-calibrated options that ensure precise liquid handling, which is essential for reproducible laboratory results. While third-party or budget brands may provide cost savings, they often compromise on calibration accuracy and build quality, potentially affecting experimental integrity.
Additionally, factors such as warranty, service support, and availability of replacement parts play a critical role in the sourcing decision. It is recommended to prioritize suppliers that offer calibration certificates, traceability, and technical support, especially for regulated or high-throughput environments. In conclusion, investing in a high-quality P200 micropipette from a trusted supplier ensures long-term performance, reduces maintenance costs, and contributes to overall laboratory efficiency and data reliability.