The global MRI equipment market is experiencing robust growth, driven by rising prevalence of chronic diseases, increasing demand for early diagnosis, and technological advancements in medical imaging. According to a report by Mordor Intelligence, the MRI market was valued at USD 7.4 billion in 2023 and is projected to grow at a compound annual growth rate (CAGR) of over 6.2% from 2024 to 2029. Similarly, Grand View Research estimates that the market size reached USD 7.6 billion in 2022 and is expected to expand at a CAGR of 5.8% through 2030, fueled by aging populations and heightened healthcare investments worldwide. As demand surges across hospitals, diagnostic centers, and research institutions, a select group of manufacturers dominate innovation, production, and global distribution. Below are the top 9 MRI manufacturers shaping the future of diagnostic imaging.
Top 9 Mri Manufacturers 2026
(Ranked by Factory Capability & Trust Score)
#1 Medical diagnostic imaging
Domain Est. 1996
Website: esaote.com
Key Highlights: Esaote is one of the world’s leading manufacturer of medical diagnostic imaging, dedicated MRI and one of the main providers for Healthcare IT. Contact us!…
#2 Magnetic Resonance Imaging (MRI) Products, Technology …
Domain Est. 1999
Website: gehealthcare.com
Key Highlights: The SIGNA™ MRI family offers a range of imaging solutions with advanced MR technology to meet your clinical needs. Choose from 1.5T, 3T, MR applications and ……
#3 Varex Imaging
Domain Est. 2016
Website: vareximaging.com
Key Highlights: Varex Imaging Corporation is the world’s largest independent supplier and manufacturer of X-ray imaging components and image processing solutions….
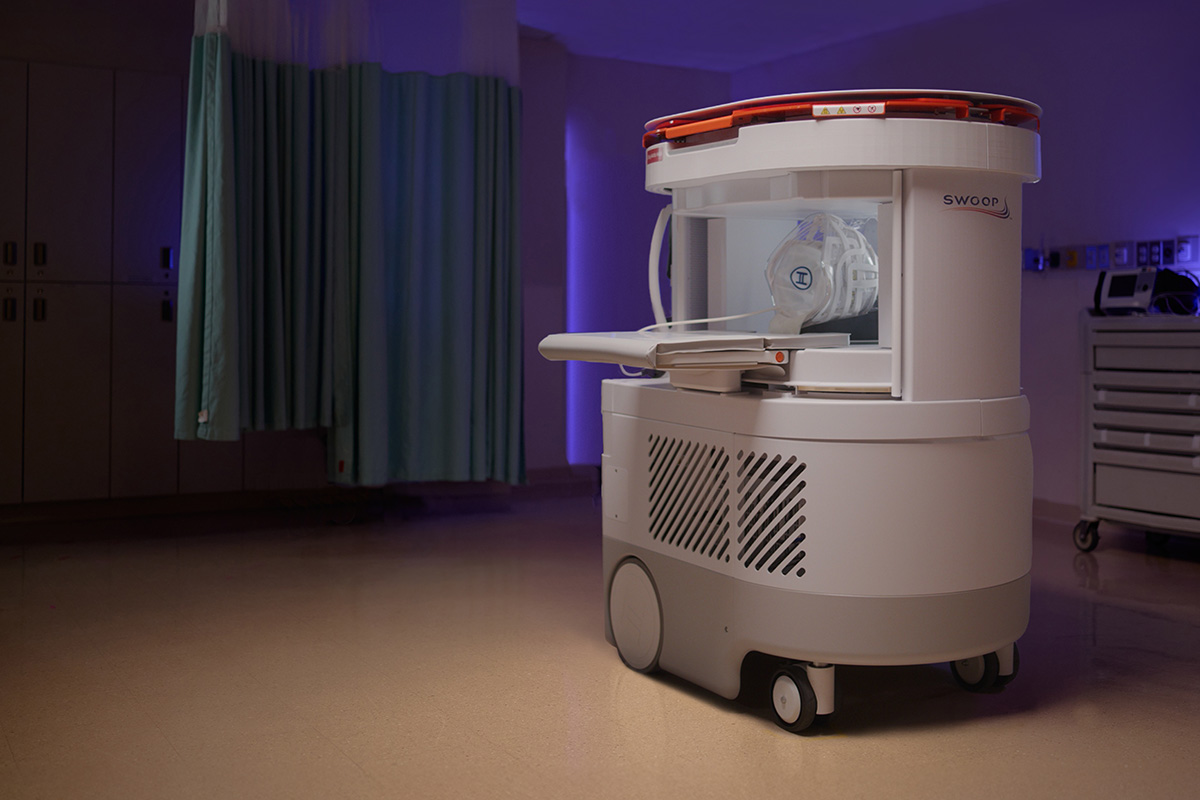
#4 Hyperfine, Inc. and the Swoop® Portable MR Imaging® System
Domain Est. 2018
Website: hyperfinemri.com
Key Highlights: The Swoop system is an AI-powered, portable brain MRI that uses a magnet with a fraction of the field strength used in conventional MRI….
#5 FONAR
Domain Est. 1995
Website: fonar.com
Key Highlights: The UPRIGHT® MRI is the only MRI scanner than can scan patients in any position, including sitting, standing, bending (for flexion and extension) or lying down….
#6 to Guerbet
Domain Est. 2000
Website: guerbet.com
Key Highlights: Guerbet has become one of the leading experts in medical imaging worldwide. Collectively, we offer a full range of medical solutions and services….
#7 United
Domain Est. 2010
Website: usa.united-imaging.com
Key Highlights: On a bold mission to provide Equal Healthcare for All™, United Imaging develops and manufactures advanced medical imaging equipment….
#8 Magnetic Resonance Imaging
Domain Est. 2016
Website: siemens-healthineers.com
Key Highlights: The definitive portfolio for MRI. Our innovative technologies enable you to achieve exceptional speed, efficiency, and precision in MRI to improve patient care ……
#9 DirectMed Expert Medical Imaging Solutions
Domain Est. 2019
Website: directmedimaging.com
Key Highlights: Fully tested, ready-to-install parts with hands-on training, service and 24/7 support for MRI coils, X-Ray equipment, ultrasounds, and more….
Expert Sourcing Insights for Mri

2026 Market Trends for MRI: Innovation, Integration, and Expansion
The global Magnetic Resonance Imaging (MRI) market is poised for significant transformation by 2026, driven by technological breakthroughs, evolving healthcare demands, and strategic industry shifts. Key trends shaping the landscape include:
Advancements in AI and Machine Learning Integration
Artificial Intelligence is rapidly moving from a supplementary tool to a core component of the MRI workflow. By 2026, expect widespread adoption of AI-powered applications for image reconstruction (enabling faster scans with lower doses), automated image analysis for lesion detection and quantification, and predictive maintenance of MRI systems. This integration will enhance diagnostic accuracy, reduce radiologist workload, and improve operational efficiency across imaging centers.
Growth of Point-of-Care and Specialized MRI Systems
The trend toward decentralized and accessible imaging is accelerating. Compact, low-field MRI systems are becoming more capable and affordable, enabling deployment in outpatient clinics, emergency departments, and even ambulances. These point-of-care solutions will expand access to MRI, particularly for musculoskeletal, neurological, and pediatric applications. Additionally, specialized MRI units—such as dedicated breast or extremity scanners—are expected to gain traction, offering optimized imaging and improved patient comfort.
Focus on Patient-Centric Design and Accessibility
Patient experience remains a critical differentiator. By 2026, manufacturers will increasingly prioritize wider bores, quieter operation, shorter scan times, and immersive environments (e.g., ambient lighting, audio-visual systems) to reduce claustrophobia and improve compliance. Efforts to make MRI more accessible to diverse populations—including bariatric patients and those with implants—will also drive design innovations and broader market penetration.
Rising Demand in Emerging Economies
Healthcare infrastructure development in regions such as Asia-Pacific, Latin America, and parts of Africa will fuel MRI market growth. Increasing government investments, rising prevalence of chronic diseases, and expanding insurance coverage are expected to boost demand for both new installations and refurbished systems. Localized service and support networks will be key to success in these high-growth markets.
Strategic Consolidation and Service-Oriented Business Models
The MRI industry is likely to see continued consolidation among manufacturers and service providers, aiming to enhance R&D capabilities and global reach. Concurrently, there will be a shift toward value-based care models, with providers increasingly adopting service contracts, pay-per-scan arrangements, and lifecycle management solutions. These models help reduce capital expenditure and ensure consistent system performance and uptime.
In summary, the MRI market in 2026 will be characterized by intelligent, accessible, and patient-focused imaging solutions, underpinned by AI and tailored to diverse clinical and geographic needs. Innovation will not only enhance diagnostic power but also expand the reach and efficiency of MRI technology worldwide.

Common Pitfalls in Sourcing MRI Services: Quality and Intellectual Property Concerns
When organizations outsource MRI (Magnetic Resonance Imaging) services—whether for medical diagnostics, research, or industrial applications—several critical pitfalls related to quality and intellectual property (IP) can undermine the value and integrity of the engagement. Being aware of these risks is essential for making informed sourcing decisions.
Quality-Related Pitfalls
Inconsistent Imaging Standards
One of the most significant quality risks is variability in imaging protocols, equipment calibration, and technician expertise across different providers. Inconsistent slice thickness, contrast usage, or field strength can lead to non-comparable or suboptimal images, especially problematic in longitudinal studies or multi-site research.
Lack of Accreditation and Compliance
Sourcing from facilities that lack proper accreditation (e.g., ACR in the U.S.) or regulatory compliance (e.g., FDA, CE marking) increases the risk of poor image quality and legal exposure. Non-compliant providers may not adhere to safety protocols or quality assurance standards.
Inadequate Radiologist Expertise
The interpretation of MRI scans requires specialized radiological training. Sourcing decisions that prioritize cost over expertise may result in misdiagnoses or missed findings, particularly in complex cases involving neurology, oncology, or musculoskeletal imaging.
Poor Data Handling and Reconstruction
Improper image reconstruction, motion artifacts, or suboptimal post-processing techniques can degrade image quality. Providers using outdated software or insufficient computational resources may deliver unusable or misleading data.
Intellectual Property-Related Pitfalls
Unclear Ownership of Imaging Data
A common IP issue arises when contracts fail to explicitly define who owns the MRI data—raw images, processed results, and derived insights. Without clear terms, the sourcing organization may lose control over critical data, limiting its ability to publish, patent, or reuse findings.
Insufficient Data Use and Licensing Agreements
Even if data ownership is assigned correctly, usage rights may be restricted. Providers might retain rights to anonymized data for research or commercial purposes unless explicitly prohibited, potentially leading to unintended data monetization or privacy breaches.
Risk of IP Leakage in Collaborative Research
In academic or industry partnerships, shared MRI data can expose proprietary methodologies or trade secrets. Without robust non-disclosure agreements (NDAs) and data segmentation protocols, sensitive IP may be inadvertently disclosed to third parties.
Compliance with Data Protection Regulations
MRI data often contains personally identifiable health information (PHI), subject to regulations like HIPAA, GDPR, or other local privacy laws. Sourcing from providers lacking appropriate data security measures can lead to breaches, regulatory penalties, and reputational damage.
Mitigation Strategies
To avoid these pitfalls, organizations should:
– Conduct thorough due diligence on provider accreditation, equipment, and staff qualifications.
– Define data ownership, usage rights, and confidentiality terms in legally binding contracts.
– Ensure compliance with relevant data protection and medical device regulations.
– Implement data quality control protocols and independent validation where feasible.
By proactively addressing quality and IP concerns, organizations can ensure reliable, secure, and legally sound MRI sourcing outcomes.

Logistics & Compliance Guide for MRI
This guide outlines key logistics and compliance considerations when shipping or handling Magnetic Resonance Imaging (MRI) equipment. Proper planning ensures safety, regulatory adherence, and timely delivery.
Regulatory Compliance
Ensure all activities comply with relevant international, national, and local regulations. Key areas include:
- Medical Device Regulations: MRI systems are classified as medical devices under regulatory frameworks such as FDA (U.S.), CE Marking (EU), and Health Canada. Verify device registration and compliance before shipment.
- Radiofrequency and Electromagnetic Standards: Confirm MRI units meet electromagnetic compatibility (EMC) standards to avoid interference with other medical or electronic systems.
- Radiation Safety: While MRI does not use ionizing radiation, strong magnetic fields require compliance with safety codes (e.g., ICNIRP guidelines). Document magnetic field safety zones and access controls.
- Export Controls: MRI systems may be subject to export control regulations (e.g., EAR in the U.S.) due to advanced technology components. Obtain required licenses for international shipments.
Transportation Logistics
MRI equipment is large, heavy, and sensitive. Logistics must ensure safe and efficient transport:
- Pre-Shipment Planning:
- Conduct site surveys to assess access routes, doorways, elevators, floor load capacity, and installation space.
- Disassemble components as needed (e.g., remove gradient coils, RF shields) following manufacturer protocols.
- Packaging:
- Use custom crating with shock absorption, moisture protection, and tamper-evident seals.
- Clearly label packages with handling instructions (e.g., “Fragile,” “This Side Up,” “Keep Dry”).
- Transport Mode:
- Use specialized heavy-lift carriers with experience in medical equipment.
- Secure magnets with active or passive shielding to prevent unintended magnetic field exposure during transit.
- Tracking and Documentation:
- Provide real-time GPS tracking for high-value shipments.
- Maintain a chain of custody log and ensure all customs documentation (e.g., commercial invoice, packing list, certificates of origin) is accurate.
Import and Customs Clearance
Smooth customs clearance requires thorough preparation:
- Harmonized System (HS) Code: Use the correct HS code for MRI machines (e.g., 9018.12 or region-specific equivalents) to determine duties and taxes.
- Duty Exemptions: Investigate eligibility for duty-free import under programs like the U.S. Medical Device Duty Exemption or WTO’s Information Technology Agreement (ITA).
- Local Agent: Appoint a licensed customs broker in the destination country to facilitate clearance and handle inspections.
- Permits and Certifications: Submit required documents such as conformity certificates, import licenses, and proof of medical use.
Installation and Site Compliance
Post-delivery activities must meet technical and safety standards:
- Magnetic Shielding: Install passive (iron) or active (electromagnetic coils) shielding to contain fringe fields and prevent interference.
- Facility Readiness: Ensure power supply (stable voltage, dedicated circuits), HVAC, RF shielding (Faraday cage), and emergency shutoff systems are operational.
- Personnel Training: Provide safety training for staff on MRI hazards (projectile effect, quench procedures, hearing protection).
- Commissioning and Testing: Perform system calibration and safety checks before clinical use. Document all tests for regulatory audits.
Environmental and Safety Considerations
- Quench Management: Plan for safe venting of helium in case of magnet quench; ensure exhaust pathways meet building and environmental codes.
- Waste Disposal: Follow environmental regulations for disposing of packaging materials and non-functional components.
- Workplace Safety: Comply with OSHA or equivalent workplace safety standards during installation and maintenance.
Recordkeeping and Audits
Maintain detailed records for compliance and traceability:
- Shipping manifests, customs documents, and delivery confirmations
- Equipment service logs and calibration records
- Staff training certifications
- Regulatory approvals and correspondence
Adhering to this logistics and compliance guide ensures the safe, legal, and efficient handling of MRI systems throughout their lifecycle. Always consult with legal, regulatory, and technical experts specific to your region and equipment.
Conclusion: Sourcing MRI Manufacturers
Sourcing MRI manufacturers requires a strategic approach that balances technological capability, regulatory compliance, cost-efficiency, and long-term support. As the demand for advanced medical imaging grows globally, selecting the right manufacturer is critical to ensuring high diagnostic accuracy, operational reliability, and patient safety. Key considerations include evaluating manufacturers’ product portfolios, innovation in imaging technology (such as high-field and portable MRI systems), adherence to international standards (FDA, CE, ISO), and after-sales service networks.
Established global leaders such as Siemens Healthineers, GE Healthcare, and Philips offer cutting-edge solutions with strong regulatory track records, while emerging players from regions like China and India present cost-effective alternatives with improving technological quality. Partnerships with manufacturers that invest in AI integration, energy efficiency, and service scalability will provide long-term value.
Ultimately, successful sourcing involves thorough due diligence, site evaluations, and pilot assessments to match organizational needs with manufacturer capabilities. A well-executed sourcing strategy not only optimizes procurement costs but also enhances diagnostic capabilities and patient care delivery in healthcare institutions.