The global laboratory equipment market, driven by increasing demand for precision instruments in pharmaceuticals, biotechnology, and clinical diagnostics, is experiencing steady growth. According to a report by Mordor Intelligence, the global laboratory equipment market was valued at USD 76.8 billion in 2023 and is projected to grow at a CAGR of 6.3% through 2029. Within this expanding landscape, microfuge racks—essential accessories designed to organize and stabilize microcentrifuge tubes during high-speed centrifugation—have gained prominence due to their role in enhancing laboratory efficiency, sample integrity, and user safety. As research output and high-throughput processing requirements rise, demand for durable, chemically resistant, and ergonomically designed microfuge racks is increasing among academic institutions, contract research organizations (CROs), and diagnostic labs. This growth is further amplified by ongoing advancements in material science and automation integration in lab workflows. Against this backdrop, a select group of manufacturers has emerged as leaders, combining innovation, scalability, and quality compliance to meet evolving laboratory standards. Below, we highlight the top 7 microfuge rack manufacturers shaping the industry based on production capacity, global reach, material innovation, and customer adoption.
Top 7 Microfuge Rack Manufacturers 2026
(Ranked by Factory Capability & Trust Score)
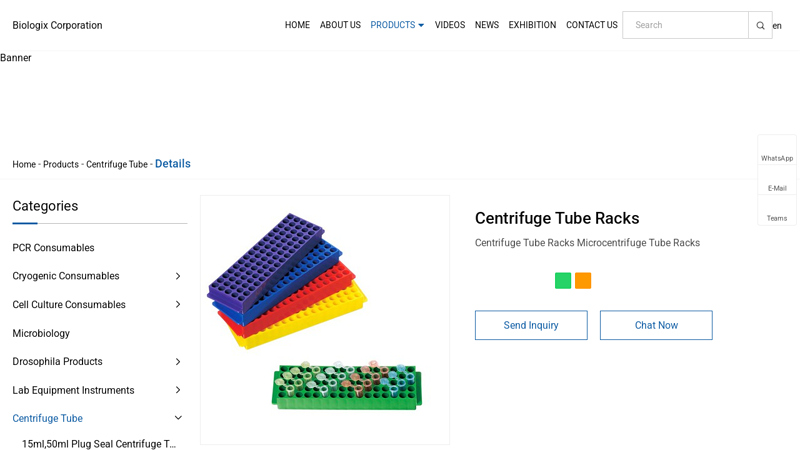
#1 Centrifuge Tube Racks Manufacturers and Suppliers
Domain Est. 2016
Website: biologixlab.com
Key Highlights: Made of high quality medical grade polypropylene. 50-well racks hold 15ml centrifuge tubes. 25-well racks hold 50ml centrifuge tubes. Sturdy design keeps tubes ……
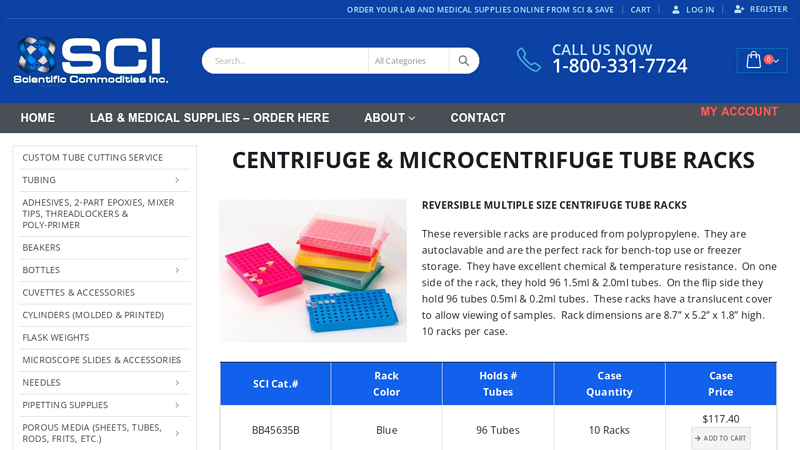
#2 Centrifuge & Microcentrifuge Tube Racks
Domain Est. 1999
Website: scicominc.com
Key Highlights: These reversible racks are produced from polypropylene. They are autoclavable and are the perfect rack for bench-top use or freezer storage….
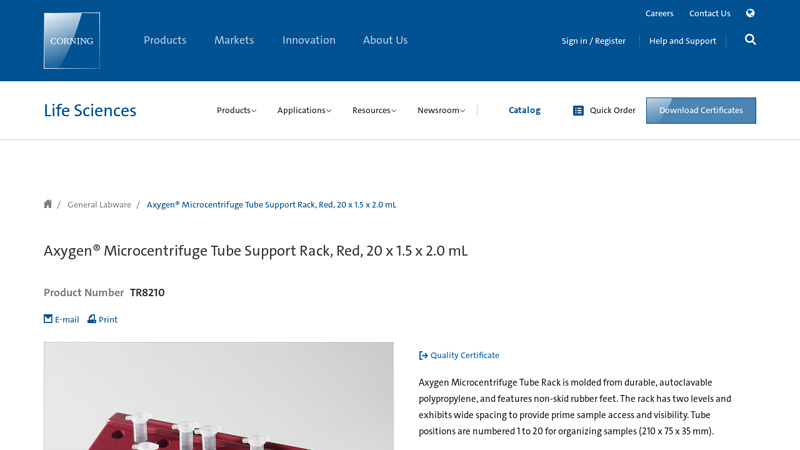
#3 Axygen® Microcentrifuge Tube Support Rack, Red, 20 x 1.5 x 2.0 mL
Domain Est. 1991
Website: ecatalog.corning.com
Key Highlights: Axygen Microcentrifuge Tube Rack is molded from durable, autoclavable polypropylene, and features non-skid rubber feet. The rack has two levels and exhibits ……
#4 Monarch® Microfuge Tube EcoRack
Domain Est. 1992
Website: neb.com
Key Highlights: In stock Free delivery over $450The Monarch Microfuge Tube EcoRack is a reversible benchtop rack made from plastic recovered during the manufacture of Monarch Purification Columns….
#5 Cole
Domain Est. 1994
Website: coleparmer.com
Key Highlights: This polypropylene (PP) racks is designed to hold 0.5mL microcentrifuge tubes; the other side holds 1.5 to 2.0mL tubes. Withstand temperatures down to -80°C (- ……
#6 Heathrow Scientific
Domain Est. 1998
Website: heathrowscientific.com
Key Highlights: Heathrow Scientific is a global supplier of laboratory equipment and supplies, providing high-quality labware for scientific research, healthcare, ……
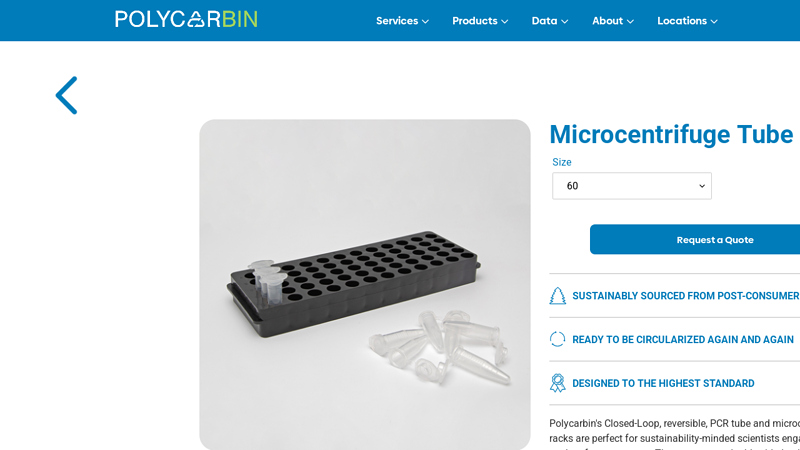
#7 Microcentrifuge Tube Racks
Domain Est. 2019
Website: polycarbin.com
Key Highlights: These compact, double-sided polypropylene racks are made from 100% circular economy resin and accommodate 0.2 mL thin-walled PCR tubes on one side….
Expert Sourcing Insights for Microfuge Rack
H2: Projected 2026 Market Trends for Microfuge Racks
The global market for microfuge racks is poised for steady growth and transformation by 2026, driven by evolving laboratory automation, increased demand in life sciences, and advancements in materials and design. These small but essential laboratory accessories play a critical role in sample organization, centrifugation safety, and workflow efficiency. Below are the key market trends expected to shape the microfuge rack industry in 2026:
1. Rising Demand in Biotechnology and Pharmaceutical Research
Ongoing investments in drug discovery, genomics, and personalized medicine are fueling the need for high-throughput laboratory processes. Microfuge racks—used extensively for organizing microcentrifuge tubes during sample preparation and storage—are seeing increased adoption in R&D laboratories. The expansion of biotech hubs in North America, Europe, and Asia-Pacific will continue to drive market demand.
2. Shift Toward Ergonomic and Lab-Integrated Designs
By 2026, manufacturers are expected to focus on ergonomic improvements, such as lightweight materials, anti-slip bases, and color-coded configurations for quick identification. Integration with automated liquid handling systems and robotic arms will also become more common, aligning microfuge racks with smart lab initiatives.
3. Growth in Sustainable and Reusable Materials
Environmental sustainability is becoming a key purchasing criterion. As laboratories aim to reduce single-use plastic waste, there will be a growing shift toward reusable, autoclavable microfuge racks made from durable polymers like polypropylene and polycarbonate. Biodegradable alternatives may also emerge as niche offerings.
4. Regional Market Expansion in Emerging Economies
Asia-Pacific, particularly countries like India, China, and South Korea, is expected to experience accelerated growth in life sciences infrastructure. Government funding for healthcare and research will increase the need for basic lab equipment, including microfuge racks. This trend will encourage both global suppliers and local manufacturers to expand their presence.
5. Customization and Specialization
The demand for specialized microfuge racks—including those designed for PCR tubes, deep-well plates, or cryogenic storage—will grow. Custom configurations for unique tube sizes and labeling compatibility will allow labs to streamline operations and reduce contamination risks.
6. E-Commerce and Direct-to-Lab Distribution Models
Digital procurement platforms will gain prominence, enabling researchers to source microfuge racks quickly and compare product specifications. Direct sales models from manufacturers will reduce costs and improve supply chain efficiency, especially for bulk institutional buyers.
7. Impact of Supply Chain Resilience
Post-pandemic, laboratories are prioritizing supply chain reliability. By 2026, manufacturers with diversified production bases and just-in-time inventory systems will have a competitive edge. This includes localized manufacturing to mitigate global disruptions.
In conclusion, the microfuge rack market in 2026 will be shaped by innovation in materials, increased automation compatibility, and a focus on sustainability and customization. As foundational tools in modern laboratories, microfuge racks will continue to evolve in response to broader shifts in scientific research and operational efficiency.

Common Pitfalls Sourcing Microfuge Rack (Quality, IP)
Sourcing microfuge racks—used to securely hold microcentrifuge tubes during laboratory procedures—requires attention to both quality and intellectual property (IP) considerations. Overlooking these aspects can lead to compromised lab performance, safety risks, or legal issues. Here are common pitfalls to avoid:
Poor Material Quality and Durability
One of the most frequent issues is selecting racks made from substandard materials. Low-quality plastics may warp under high temperatures (e.g., during autoclaving), become brittle over time, or leach contaminants into sensitive samples. This compromises sample integrity and equipment longevity. Always verify that the material (e.g., polypropylene) is chemically resistant, autoclavable (typically up to 121°C), and certified for laboratory use.
Inconsistent Dimensional Tolerances
Inferior manufacturing processes can result in racks with inconsistent hole sizes or spacing. This leads to tubes not fitting properly—either too loose (risking spills) or too tight (making tube insertion/removal difficult). Poor tolerances also prevent compatibility with automated systems or centrifuge rotors, disrupting workflows. Ensure racks meet precise dimensional standards and are tested for compatibility with common tube types (e.g., 1.5 mL and 2.0 mL microcentrifuge tubes).
Lack of Design Validation and Certification
Many generic rack suppliers do not provide documentation proving design validation, biocompatibility, or sterility. Using uncertified racks in regulated environments (e.g., diagnostics, pharmaceuticals) may violate quality management standards such as ISO 13485 or GLP. Always request certificates of conformance, material safety data sheets (MSDS), and, if applicable, DNA/RNase-free validation.
Intellectual Property (IP) Infringement Risks
Copying the design of branded microfuge racks—such as those from major labware manufacturers—can expose buyers and suppliers to IP violations. Patented features may include unique geometries, labeling systems, or ergonomic designs. Sourcing from suppliers that reverse-engineer protected products can lead to legal disputes, shipment seizures, or reputational damage. Conduct due diligence to ensure designs are either licensed, in the public domain, or independently developed without infringement.
Inadequate Traceability and Supplier Reliability
Unreliable suppliers may lack batch traceability, making it difficult to investigate contamination events or quality failures. Sudden discontinuation of supply or inconsistent quality between batches can disrupt lab operations. Establish long-term relationships with reputable manufacturers who provide lot numbers, quality control reports, and stable supply chains.
Overlooking Ergonomic and Safety Features
Poorly designed racks may lack features like labeling areas, color-coding, or anti-slip bases, increasing the risk of sample mix-ups or accidents. Racks with sharp edges or unstable stacking can endanger lab personnel. Prioritize designs that enhance usability and safety, especially in high-throughput environments.
By proactively addressing these quality and IP pitfalls, organizations can ensure reliable, compliant, and safe laboratory operations when sourcing microfuge racks.

Logistics & Compliance Guide for Microfuge Rack
Product Overview
The Microfuge Rack is a laboratory tool designed for the organized storage and transport of microcentrifuge tubes (typically 0.2 mL to 2.0 mL). Constructed from durable, chemically resistant materials such as polypropylene or autoclavable plastics, it supports efficient sample handling in research, clinical, and diagnostic environments. Proper logistics and compliance procedures are essential to ensure product integrity, safety, and regulatory adherence.
Regulatory Compliance
Microfuge Racks must comply with relevant international and regional standards to ensure safety, quality, and traceability. Key compliance requirements include:
- ISO 13485: If used in medical device manufacturing or testing environments, racks may need to be part of a quality management system compliant with ISO 13485.
- USP Class VI Certification: For racks used in pharmaceutical or biomedical applications, biocompatibility as per USP <87> and <88> may be required.
- REACH & RoHS: Compliance with EU regulations on restricted hazardous substances (RoHS) and chemical registration (REACH) is mandatory for products distributed in Europe.
- FDA Registration: Facilities manufacturing or distributing racks used in clinical settings may need to be registered with the U.S. Food and Drug Administration, especially if used as components in IVD (In Vitro Diagnostic) workflows.
Manufacturers should provide a Certificate of Compliance (CoC) or Declaration of Conformity (DoC) upon request.
Packaging & Labeling Requirements
To maintain sterility, prevent contamination, and ensure traceability, Microfuge Racks must be appropriately packaged and labeled:
- Sterile Racks: Must be individually or bulk-packaged in sterile barrier systems (e.g., Tyvek pouches) and labeled with sterilization method (e.g., gamma irradiation), lot number, expiration date, and sterility claim.
- Non-Sterile Racks: Should be packed in sealed poly bags or corrugated cartons to protect against dust and moisture.
- Labeling: All packages must include product name, catalog number, quantity, material composition, manufacturer details, batch/lot number, and handling instructions (e.g., “Do Not Autoclave” if not applicable).
- Hazard Communication: If the rack contains substances of concern (e.g., certain plastics with additives), labeling under GHS (Globally Harmonized System) may be required.
Storage Conditions
Proper storage ensures product performance and longevity:
- Store in a clean, dry environment at temperatures between 15°C and 30°C.
- Avoid direct sunlight and exposure to UV radiation to prevent material degradation.
- Keep away from strong oxidizing agents, organic solvents, and corrosive chemicals.
- Stack cartons no higher than recommended to prevent crushing.
Shelf life for sterile racks is typically 3–5 years; refer to manufacturer specifications.
Transportation Guidelines
Ensure safe and compliant shipment of Microfuge Racks:
- Use sturdy, recyclable packaging materials to prevent damage during transit.
- For international shipments, comply with IATA (air) or IMDG (sea) regulations if transporting sterile or chemically treated items.
- Include proper shipping documentation: commercial invoice, packing list, and certificates of compliance if required.
- Temperature-controlled transport is not typically required unless specified (e.g., for pre-sterilized or temperature-sensitive variants).
- Avoid freezing or exposure to extreme heat during transport.
Handling & Usage Compliance
End-users must adhere to best practices:
- Inspect packaging for damage before use; discard if compromised.
- Follow manufacturer instructions for autoclaving (typically 121°C, 15–20 psi, 20 minutes for polypropylene racks). Not all racks are autoclavable—verify before sterilization.
- Do not exceed recommended tube capacity to prevent warping or sample spillage.
- Clean with mild detergents and water; avoid abrasive cleaners or strong solvents unless specified.
Environmental & Disposal Compliance
- Microfuge Racks made from polypropylene (PP) or polycarbonate (PC) are recyclable in appropriate waste streams (check local regulations).
- Contaminated racks (e.g., with biohazardous or chemical residues) must be decontaminated following institutional biosafety protocols before disposal.
- Comply with local, state, and federal waste regulations (e.g., EPA, OSHA, or EU WEEE directives if applicable).
Documentation & Traceability
Maintain full traceability through:
- Batch/lot tracking for quality control and recalls.
- Retention of supplier CoC/DoC for audit purposes.
- Internal inventory logs noting receipt date, storage location, and usage.
For GLP- or GMP-regulated environments, document rack use in experimental records as part of equipment control.
Conclusion
Adherence to logistics and compliance protocols ensures the safe, effective use of Microfuge Racks in regulated environments. Always consult the manufacturer’s instructions and institutional biosafety guidelines to maintain compliance with evolving regulatory standards.
Conclusion for Sourcing Microfuge Rack:
After evaluating various suppliers, materials, compatibility, durability, and cost-effectiveness, the sourcing of microfuge racks should prioritize quality and functionality to ensure laboratory efficiency and sample integrity. Opting for autoclavable, chemically resistant racks made from durable materials such as polypropylene or stainless steel ensures long-term usability and compliance with sterile working conditions. Additionally, selecting racks that accommodate standard microcentrifuge tube sizes (e.g., 1.5 mL and 2.0 mL) with secure tube retention minimizes the risk of contamination or sample spillage. Supplier reliability, lead times, and volume pricing should also be considered to support consistent inventory and operational needs. Ultimately, investing in well-designed, ergonomically optimized microfuge racks from reputable suppliers enhances laboratory organization, safety, and productivity.