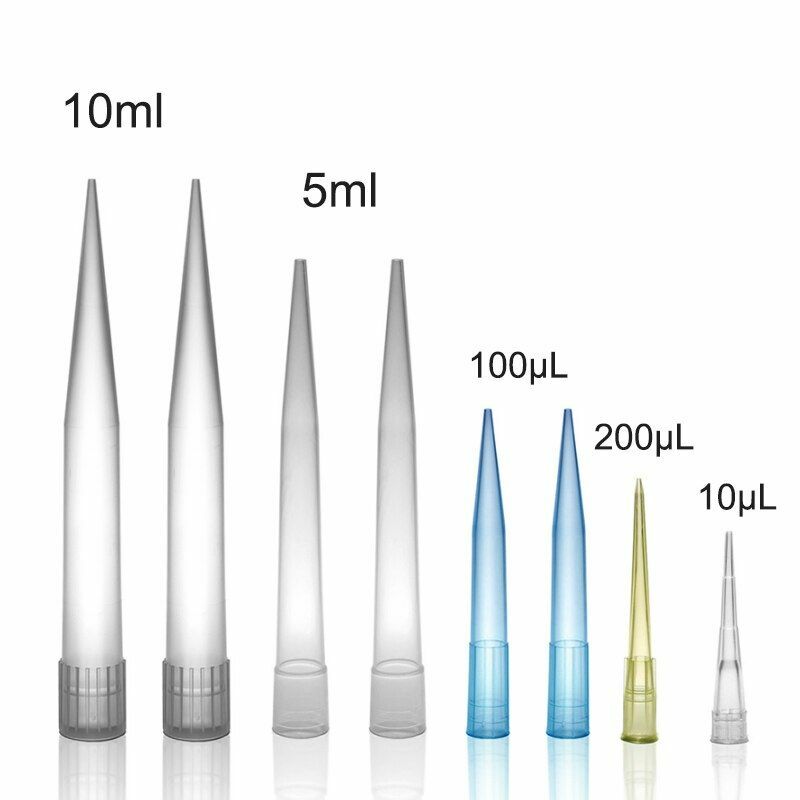
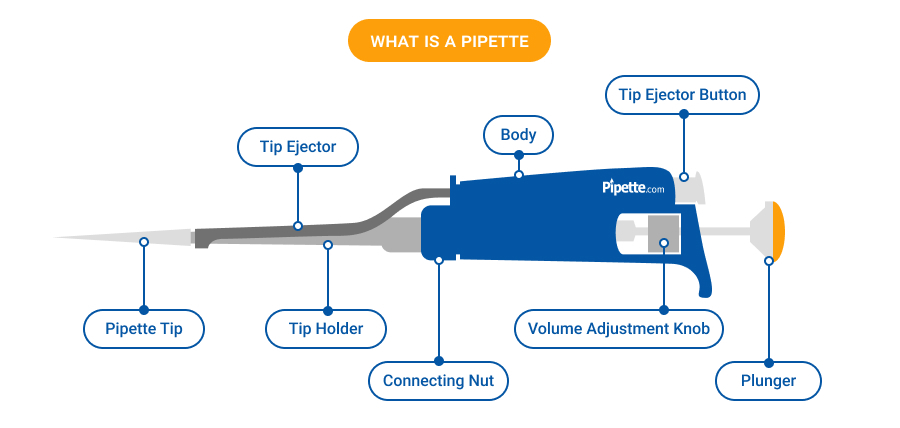
The global micro pipette tips market is experiencing steady growth, driven by increasing demand in life sciences, pharmaceuticals, and academic research. According to Grand View Research, the global laboratory plastics market—of which micro pipette tips are a critical component—was valued at USD 9.8 billion in 2022 and is expected to expand at a compound annual growth rate (CAGR) of 6.8% from 2023 to 2030. This growth is fueled by rising R&D investments, automation in laboratories, and the expansion of biotechnology and drug discovery initiatives worldwide. With precision, contamination control, and compatibility being paramount, laboratories are increasingly focused on sourcing high-quality micro pipette tips from reliable manufacturers. As demand intensifies, the competitive landscape has evolved, shaping a market dominated by innovators committed to volume accuracy, low retention surfaces, and sustainability. Based on market presence, product quality, and technological advancement, the following eight companies have emerged as leading micro pipette tips manufacturers meeting the rigorous standards of modern laboratories.
Top 8 Micro Pipette Tips Manufacturers 2026
(Ranked by Factory Capability & Trust Score)
#1 Pipette Tips, Pipettors, and Accessories
Domain Est. 1991
Website: corning.com
Key Highlights: We specialize in manufacturing high quality pipette tips for all major automation platforms, for consistency and reliability in small-volume liquid handling….
#2 Micro Pipette Tips
Domain Est. 1993
Website: mt.com
Key Highlights: Rainin Pipette Tips provide excellent clarity and flexibility for accurate touch-off. Rainin pipette tips are available in LTS and universal formats….
#3 Eppendorf Pipette Tips
Domain Est. 1995
Website: eppendorf.com
Key Highlights: In stockepT.IPS pipette tips work in perfect harmony with your Eppendorf pipettes! The ergonomically designed cone geometry allows for complete sealing….
#4 Pipette Tips
Domain Est. 1995
Website: fishersci.com
Key Highlights: A science education product. Racked Filter Micropipette Tips are disposable tips intended for use with most brands of pipettors. 960 Pk….
#5 Pipetting
Domain Est. 1996
Website: sartorius.com
Key Highlights: Pipette Tips. Our Optifit and Safetyspace® Filter Tips are designed to perfectly match our pipettes, permitting maximum accuracy, precision and ergonomics….
#6 TipOne Pipette Tips
Domain Est. 1998
Website: usascientific.com
Key Highlights: TipOne is the original easy-to-load, refillable tip system that saves space and reduces waste. They are reusable, easy to refill, and will last through repeated ……
#7 Pipette.com – your go
Domain Est. 2000
Website: pipette.com
Key Highlights: Pipette.com is your go-to scientific supplies company providing high-quality laboratory consumables and equipment for more than 25 years….
#8 Pipette Tips
Domain Est. 2007
Website: celltreat.com
Key Highlights: Free deliveryCELLTREAT Scientific Products Pipette Tips deliver high performance with exceptional value. A perfected balance between raw materials and efficient ……
Expert Sourcing Insights for Micro Pipette Tips

H2: 2026 Market Trends for Micro Pipette Tips
The global micro pipette tips market is poised for steady growth through 2026, driven by advancements in life sciences, rising demand for precision in laboratory workflows, and expanding applications in diagnostics, pharmaceuticals, and academic research. Several key trends are expected to shape the market landscape during this period.
-
Increased Demand for High-Precision and Low-Retention Tips
As research methodologies become more sensitive—particularly in genomics, single-cell analysis, and high-throughput screening—there is a growing preference for ultra-precise, low-binding pipette tips. These tips minimize sample retention and contamination, ensuring higher accuracy and reproducibility. By 2026, manufacturers are expected to focus on surface-treated and specialty-coated tips (e.g., filtered, aerosol-resistant) to meet stringent regulatory and performance standards. -
Rising Adoption of Automated Liquid Handling Systems
Automation in laboratories is accelerating, especially in drug discovery and clinical diagnostics. This shift increases demand for pipette tips compatible with robotic platforms and multi-channel pipettors. OEM partnerships between tip manufacturers and automation companies will likely expand, with a focus on standardized, high-volume consumables designed for seamless integration. -
Sustainability and Eco-Friendly Materials
Environmental concerns are reshaping product development. By 2026, the market will see a stronger push toward sustainable manufacturing, including bio-based plastics (e.g., PLA) and recyclable tip racks. Leading vendors are expected to offer “green” product lines to comply with institutional sustainability goals and reduce plastic waste in research facilities. -
Regional Market Expansion
While North America and Europe remain dominant due to well-established research infrastructure, the Asia-Pacific region—especially China, India, and South Korea—is projected to experience the fastest growth. Increased government funding for biotechnology, expansion of contract research organizations (CROs), and rising investments in healthcare R&D will drive regional demand. -
Supply Chain Resilience and Localization
Post-pandemic lessons have emphasized the need for robust supply chains. By 2026, manufacturers are anticipated to diversify production bases and increase regional manufacturing to mitigate disruptions. Nearshoring and onshoring strategies may become more prevalent, especially in North America and Europe. -
Innovation in Smart and Connected Consumables
Emerging trends include the development of traceable or “smart” pipette tips embedded with RFID or QR codes for inventory management and quality control. While still in early stages, this technology could gain traction in regulated environments such as GLP and GMP labs by 2026.
In conclusion, the micro pipette tips market in 2026 will be characterized by innovation, sustainability, and integration with digital and automated workflows. Companies that prioritize product differentiation, environmental responsibility, and supply chain agility are likely to gain competitive advantage in this evolving landscape.

Common Pitfalls Sourcing Micro Pipette Tips (Quality, IP)
When sourcing micro pipette tips—especially for sensitive laboratory applications—overlooking key quality and intellectual property (IP) concerns can lead to compromised results, regulatory issues, and legal risks. Below are common pitfalls to avoid:
Quality Inconsistencies and Compatibility Issues
Many low-cost or generic pipette tips suffer from inconsistent manufacturing standards. Variations in tip geometry, internal volume, and fit can lead to inaccurate pipetting, aspiration errors, or tip ejection problems. Poorly molded tips may also introduce contaminants such as endotoxins, RNase, DNase, or leachable chemicals that affect sensitive assays. Additionally, tips not designed for specific pipette models may cause leaks or incomplete seals, undermining data reliability and reproducibility.
Lack of Traceability and Certification
Reputable suppliers provide lot-specific documentation, including sterility certifications, absence of nucleases, and compliance with ISO standards. Sourcing from vendors without proper traceability increases the risk of using substandard products, especially in regulated environments such as clinical diagnostics or pharmaceutical development. Without verifiable quality control data, labs may unknowingly compromise experiment integrity.
Intellectual Property (IP) Infringement Risks
Many branded pipette tips (e.g., those designed for Gilson, Eppendorf, or Thermo Fisher systems) are protected by design patents or utility patents. Sourcing “compatible” or “universal” tips from manufacturers that replicate patented designs without licensing can expose buyers to IP infringement claims. While some generic tips are legally produced under fair-use or expired patents, others may violate intellectual property rights, potentially leading to legal action, supply chain disruptions, or seizure of goods.
Misrepresentation of Compatibility and Performance
Some suppliers advertise tips as compatible with major pipette brands without rigorous testing. In reality, these tips may not meet the dimensional or functional specifications required for accurate performance. Misleading claims about accuracy, precision, or filtration capabilities (e.g., aerosol barrier tips) can result in failed experiments or contamination events, particularly in PCR, cell culture, or high-throughput screening applications.
Supply Chain and Vendor Reliability
Relying on unknown or offshore suppliers may lead to inconsistent delivery, long lead times, or sudden discontinuation of products. Lack of direct technical support makes troubleshooting difficult. Furthermore, inconsistent quality across production batches undermines long-term experimental reproducibility, especially in multi-site or longitudinal studies.
To mitigate these risks, laboratories should source pipette tips from reputable suppliers with transparent quality control processes, clear IP compliance, and documented compatibility testing. Conducting in-house validation and maintaining records of supplier certifications can safeguard both data integrity and legal compliance.

Logistics & Compliance Guide for Micro Pipette Tips
Product Classification and Regulatory Overview
Micro pipette tips are generally classified as laboratory consumables and may fall under different regulatory categories depending on their intended use, material composition, and region. In most jurisdictions, they are considered general lab supplies unless specifically designed for diagnostic or medical purposes. However, if used in clinical diagnostics or regulated testing (e.g., under CLIA, FDA 510(k), or IVD directives), compliance with applicable standards becomes essential.
International Trade and Customs Classification
Micro pipette tips are typically classified under the Harmonized System (HS) code 3926.30 – “Articles of plastics, for technical uses.” Specific sub-codes may vary by country. Accurate classification is critical for customs clearance, duty assessment, and import/export compliance. Documentation should include:
– Commercial invoice with detailed product description
– Packing list
– Certificate of Origin (if required)
– Material Safety Data Sheet (MSDS/SDS), if applicable
Packaging and Shipping Requirements
- Packaging: Tips must be shipped in sealed, sterile (if required), and contamination-free packaging. Racks or multichannel tip boxes should be protected with cushioning to prevent damage during transit.
- Labeling: Include product name, lot number, expiration date (if applicable), sterility status, quantity, and manufacturer details. For sterile tips, clearly indicate sterilization method (e.g., gamma irradiation).
- Shipping: Non-sterile tips may be shipped via standard courier; sterile tips require temperature-controlled or protected transport to maintain integrity. Avoid extreme temperatures and humidity.
Regulatory Compliance by Region
United States (FDA & EPA)
– No FDA pre-market approval is required for general lab use tips.
– If used in clinical testing, compliance with CLIA and GLP standards may apply.
– EPA regulations may apply if tips contain regulated substances (e.g., certain plastics or additives).
European Union (REACH & RoHS)
– Comply with REACH (EC 1907/2006) for chemical substances in plastics (e.g., phthalates).
– Adhere to RoHS (2011/65/EU) if electrical components are involved (rare for tips).
– CE marking not typically required unless part of a medical device system.
ISO Standards
– ISO 8655 – Specifies requirements for piston-operated volumetric apparatus, including compatibility and performance of pipette tips.
– ISO 13485 – Relevant if tips are manufactured for medical device applications.
– ISO 10993 – Required if tips contact biological samples and biocompatibility claims are made.
Environmental and Disposal Compliance
- Most micro pipette tips are made from polypropylene (PP), which is recyclable in some facilities.
- Follow local regulations for lab waste disposal.
- Biohazardous tips (used with infectious agents) must be disposed of as biohazard waste per OSHA and CDC guidelines.
- Ensure compliance with WEEE (EU) or RCRA (US) if applicable.
Quality Assurance and Documentation
- Maintain records of lot traceability, sterilization (if applicable), and quality control testing.
- Suppliers should provide Certificates of Analysis (CoA) and Compliance (CoC).
- Regular audits of manufacturing and distribution partners are recommended to ensure ongoing compliance.
Import/Export Licensing
- Generally, no special license is required for micro pipette tips.
- Exceptions may apply if exported to embargoed countries or if tips are part of a regulated diagnostic kit.
- Verify requirements with customs authorities in destination country.
Best Practices for Supply Chain Management
- Partner with certified suppliers adhering to ISO 9001 or equivalent.
- Monitor regulatory updates in target markets.
- Implement inventory controls to prevent use of expired or compromised products.
- Train staff on proper handling, storage, and compliance protocols.
By following this guide, laboratories and distributors can ensure the safe, legal, and efficient handling of micro pipette tips across global supply chains.
Conclusion for Sourcing Micro Pipette Tips
Sourcing micro pipette tips requires careful consideration of several key factors to ensure accuracy, reproducibility, and efficiency in laboratory workflows. Compatibility with existing pipetting instruments, tip quality (such as precision, sterility, and low retention properties), and consistency across batches are critical to maintaining experimental integrity. Additionally, evaluating suppliers based on reliability, cost-effectiveness, and adherence to sustainability practices—such as recyclable packaging or low-plastic alternatives—can support both scientific and environmental goals.
By balancing performance requirements with budgetary and ethical considerations, laboratories can establish a robust sourcing strategy that enhances operational efficiency, reduces contamination risks, and supports long-term research objectives. Ultimately, investing time in selecting the right pipette tips from reputable suppliers contributes significantly to the reliability and success of laboratory outcomes.