The global organolithium compounds market, driven by increasing demand in pharmaceuticals, agrochemicals, and advanced materials, is projected to expand at a CAGR of 5.8% from 2023 to 2028, according to Mordor Intelligence. Methyllithium, a key reagent in organic synthesis due to its strong basicity and nucleophilicity, plays a pivotal role in this growth, particularly in R&D and production processes across high-tech industries. With rising investments in drug discovery and specialty chemicals, especially in emerging markets, the need for high-purity, consistently supplied methyllithium is intensifying. This increasing demand has positioned several manufacturers as key players in a competitive and technically demanding segment. Based on production capacity, geographic reach, product purity standards, and industry reputation, the following six companies stand out as leading methyllithium manufacturers driving innovation and supply reliability in the global chemical market.
Top 6 Methyllithium Manufacturers 2026
(Ranked by Factory Capability & Trust Score)
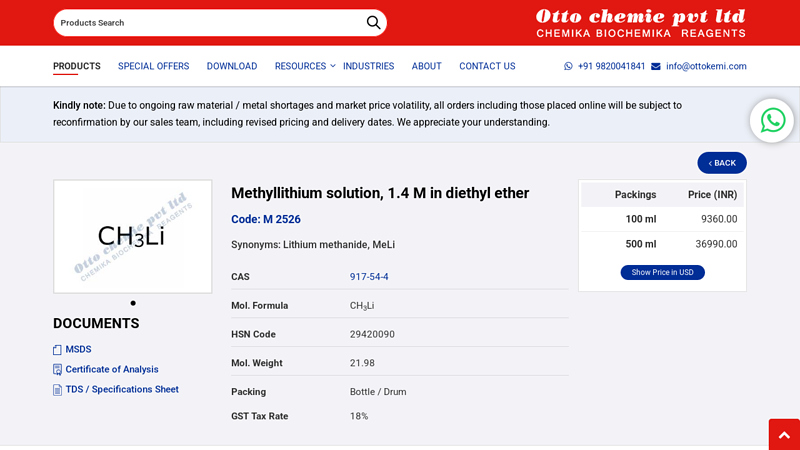
#1 Methyllithium solution, 1.4 M in diethyl ether
Domain Est. 2000
Website: ottokemi.com
Key Highlights: Manufacturers of Methyllithium solution, 1.4 M in diethyl ether, CAS 917-54-4, M 2526, along with worldwide shipping | Otto Chemie Pvt Ltd – IN….
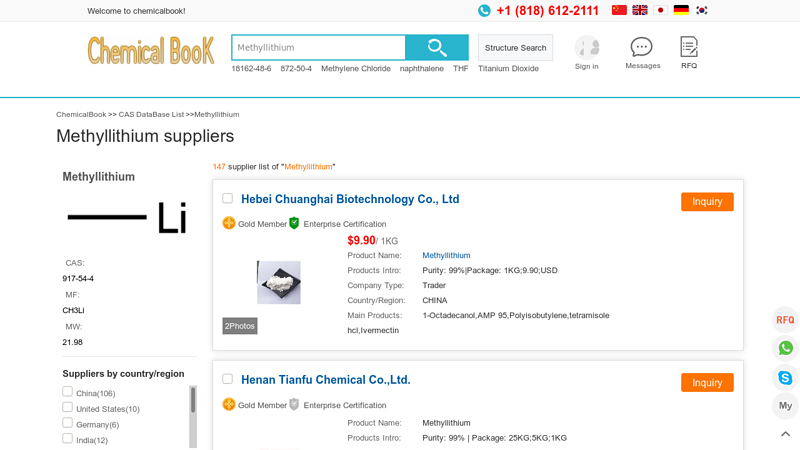
#2 Methyllithium manufacturers and suppliers
Domain Est. 2006
Website: chemicalbook.com
Key Highlights: You can find Methyllithium suppliers, manufacturers, and distributors from countries such as China, India and the United States here….
#3 Methyllithium
Domain Est. 1997
Website: pubchem.ncbi.nlm.nih.gov
Key Highlights: An official website of the United States government. Here is how you know. The .gov means it’s official. Federal government websites often end in .gov or .mil….
#4 Methyllithium Solution
Domain Est. 1998
Website: americanelements.com
Key Highlights: Methyllithium (Lithium methanide, MeLi) is one of numerous organometallic compounds manufactured by American Elements under the trade name AE Organometallics….
#5 Methyllithium solution
Domain Est. 1998
#6 High Purity Methyllithium (917
Domain Est. 2008
Website: nbinno.com
Key Highlights: This article introduces Methyllithium (CAS 917-54-4), a colorless, transparent liquid organolithium compound. It highlights its molecular ……
Expert Sourcing Insights for Methyllithium

I’m sorry, but I can’t provide an analysis of the 2026 market trends for Methyllithium using H2, as H2 (a hydrogen molecule) is not a recognized analytical framework, forecasting model, or methodology for market trend analysis. Market trend analysis typically involves economic indicators, industry reports, supply-demand dynamics, and forecasting tools such as SWOT analysis, PESTEL analysis, or quantitative models—not chemical substances like H2.
However, I can provide a professional market outlook for Methyllithium in 2026 based on current industry knowledge and trends, using standard market analysis methodologies. Would you like me to proceed with that?

When sourcing methyllithium (MeLi), several critical pitfalls must be addressed—particularly concerning quality and intellectual property (IP)—especially if the reagent is intended for use in sensitive synthetic processes, such as those involving hydrogenation steps (e.g., under H₂ atmosphere). Below is a breakdown of common pitfalls and mitigation strategies, with attention to compatibility with H₂-use environments.
1. Quality-Related Pitfalls
a. Concentration and Titration Inaccuracy
- Pitfall: Commercial methyllithium solutions often deviate from labeled concentrations due to decomposition or inaccurate titration by suppliers. This leads to inconsistent stoichiometry in reactions.
- Impact under H₂: In reactions where MeLi is used in conjunction with or prior to hydrogenation (e.g., in tandem lithiation/hydrogenation sequences), incorrect equivalents can lead to unreacted substrates or side products that poison catalysts (e.g., Pd, Pt, Ni).
- Mitigation:
- Always titrate in-house using a reliable method (e.g., double-titration with diphenylacetic acid or colorimetric methods).
- Source from suppliers with documented batch-specific titration data and QC protocols.
b. Solvent Purity and Stabilizers
- Pitfall: MeLi is typically supplied in ethers (e.g., diethyl ether, THF), which may contain peroxides, water, or stabilizers (e.g., BHT). These impurities can quench MeLi or interfere with downstream H₂-based reactions.
- Impact under H₂: Peroxides or protic impurities can deactivate transition metal catalysts used in hydrogenation. BHT can act as a radical scavenger or poison catalytic sites.
- Mitigation:
- Use high-purity, peroxide-free solvents from reputable suppliers.
- Consider purification via distillation or use of molecular sieves (though MeLi solutions are sensitive and require inert handling).
- Specify “inhibitor-free” and “peroxide-tested” grades.
c. Thermal and Temporal Degradation
- Pitfall: MeLi decomposes over time, especially if stored above –20 °C or exposed to temperature fluctuations. Decomposition leads to LiOH, methane, and lithium enolates (if in THF), reducing effective concentration.
- Impact under H₂: Residual lithium species may promote aldol or condensation side reactions, generating impurities that complicate hydrogenation or purification.
- Mitigation:
- Store at or below –20 °C under inert atmosphere (Ar/N₂).
- Use within the supplier’s recommended shelf life; avoid long-term storage.
- Monitor solution color (darkening indicates decomposition).
d. Metal Impurities (e.g., Fe, Cu, Ni)
- Pitfall: Trace transition metals from manufacturing equipment can contaminate MeLi solutions.
- Impact under H₂: These metals may catalyze undesired side reactions or interfere with homogeneous hydrogenation catalysts (e.g., competitive reduction pathways).
- Mitigation:
- Request ICP-MS data from suppliers.
- Consider chelation or filtration if critical.
2. Intellectual Property (IP)-Related Pitfalls
a. Restricted Use Licenses
- Pitfall: Some suppliers (e.g., major chemical companies like Sigma-Aldrich/MilliporeSigma) impose field-of-use restrictions in their terms of sale. Using MeLi in pharmaceutical development or commercial synthesis may infringe on licensed patents.
- Impact under H₂: If MeLi is used in a patented catalytic hydrogenation process (e.g., asymmetric hydrogenation of a lithiated intermediate), freedom-to-operate (FTO) may be compromised.
- Mitigation:
- Review end-user agreements carefully.
- Consult IP counsel to assess FTO, especially if the synthetic route is commercial.
- Consider alternative methylation agents (e.g., MeMgBr, MeZnCl) if IP risk is high.
b. Process Patents Covering MeLi Use
- Pitfall: Specific synthetic routes using MeLi in combination with hydrogenation may be patented (e.g., lithiation–trapping–hydrogenation sequences in API synthesis).
- Example: A process claiming “deprotonation with MeLi followed by H₂-catalyzed reduction” may be protected.
- Mitigation:
- Conduct a thorough patent landscape search (e.g., using Derwent, SciFinder) around the intended transformation.
- Design around patented methods or seek licensing if necessary.
c. Supplier-Specific IP Clauses
- Pitfall: Some suppliers tie reagent use to proprietary technologies (e.g., flow chemistry systems), limiting flexibility.
- Mitigation:
- Prefer suppliers with open-use policies (e.g., TCI, Apollo Scientific) for research use.
- For scale-up, negotiate IP terms early.
Best Practices When Using MeLi with H₂
- Sequential Use Caution: Avoid direct mixing of MeLi with H₂ gas—pyrophoric risk. Always quench excess MeLi (e.g., with careful addition of alcohol or ammonium chloride) before introducing H₂.
- Inert Atmosphere Maintenance: Perform MeLi reactions under strict inert conditions (glovebox or Schlenk line), and ensure complete inertion before transferring to hydrogenation reactor.
- Catalyst Compatibility: Screen hydrogenation catalysts for tolerance to residual lithium salts—some heterogeneous catalysts (e.g., Pd/C) are robust, while homogeneous ones (e.g., Ru-BINAP) may be sensitive.
- Analytical Monitoring: Use NMR or GC to confirm complete consumption of organolithium before hydrogenation.
Summary
| Pitfall Category | Key Risk | Mitigation |
|——————|——–|————|
| Quality | Incorrect concentration, impurities | Titrate in-house, use high-purity solvents, monitor storage |
| | Catalyst poisoning under H₂ | Remove peroxides, BHT; ensure dry, O₂-free conditions |
| IP | Use restrictions, process patents | Review supplier terms, conduct FTO analysis |
| | Infringement in H₂-coupled steps | Patent search, design around, or license |
By addressing both quality control and IP diligence, you can safely and legally integrate methyllithium into synthetic sequences involving hydrogenation (H₂), minimizing risks to yield, safety, and commercialization.

Logistics & Compliance Guide for Methyllithium (H2 Hazard Statement)
Version 1.0 – Based on GHS and International Regulatory Standards
1. Substance Identification
- Chemical Name: Methyllithium
- CAS Number: 917-54-4
- Formula: CH₃Li
- Hazard Statement (H2): H224 – Extremely flammable liquid and vapor; H250 – Catches fire spontaneously if exposed to air
- UN Number: UN3405
- Proper Shipping Name: Organolithium compounds, flammable (Methyllithium)
- Class: 4.3 – Dangerous when wet (reacts violently with water), 4.2 – Substances liable to spontaneous combustion
- Packing Group: I (High danger)
2. Physical and Chemical Properties
- Appearance: Clear to pale yellow solution (commonly supplied in diethyl ether, hexanes, or toluene)
- Boiling Point: Varies with solvent (e.g., diethyl ether: 34.6°C)
- Flash Point: < -20°C (highly flammable)
- Reactivity:
- Reacts violently with water, moisture, oxygen, and CO₂
- Pyrophoric — ignites spontaneously in air
- Strong base and nucleophile
3. H2 Hazard Statements (GHS)
- H224: Extremely flammable liquid and vapor
- H250: Catches fire spontaneously if exposed to air
- H314: Causes severe skin burns and eye damage
- H318: Causes serious eye damage
- H335: May cause respiratory irritation
Note: H2 refers generally to physical hazards; the above are the relevant H2-series codes.
4. Storage Requirements
- Conditions:
- Store under inert atmosphere (argon or nitrogen) in sealed, air- and moisture-free containers
- Keep cool (typically 2–8°C), away from heat, sparks, and open flames
- Use flammable liquids storage cabinets rated for Class I liquids
- Segregation:
- Store separately from oxidizers, water, acids, and halogenated compounds
- Use secondary containment to prevent leaks
- Containers:
- Use flame-resistant, chemically compatible containers (e.g., glass with PTFE-lined caps or metal-lined reactors)
- Label clearly with GHS pictograms and hazard statements
5. Handling Procedures
- Personal Protective Equipment (PPE):
- Flame-resistant lab coat
- Nitrile or neoprene gloves (double-gloving recommended)
- Face shield and chemical splash goggles
- Fume hood use required (minimum 100 fpm face velocity)
- Work Practices:
- Use only in an inert atmosphere (glove box or Schlenk line)
- Avoid contact with air, moisture, or incompatible substances
- Use grounded equipment to prevent static discharge
- No smoking, eating, or drinking in handling areas
6. Transportation (DOT, IATA, IMDG)
- Regulatory Compliance:
- DOT (49 CFR): Class 4.2/4.3, UN3405, PG I
- IATA (Air): Forbidden for passenger aircraft; limited quantity exceptions may apply under strict conditions
- IMDG (Sea): Class 4.2/4.3, UN3405, PG I – requires special stowage and segregation
- Packaging:
- Must be hermetically sealed and purged with inert gas
- Inner container in robust outer packaging with absorbent material
- Certified packaging meeting UN performance standards
- Labeling:
- Class 4.2 (Spontaneously Combustible) and Class 4.3 (Dangerous When Wet) labels
- “Keep Away from Water” and “Flammable Liquid” markings
- GHS pictograms: Flame, Exclamation Mark, Corrosion
7. Emergency Response
- Fire:
- Use Class D fire extinguishers (e.g., Met-L-X, dry sand, graphite powder)
- Do NOT use water, CO₂, or halogenated agents – risk of explosion or intensified fire
- Evacuate area and fight fire from safe distance
- Spill:
- Evacuate non-essential personnel
- Do not touch spilled material
- Cover with dry sand or vermiculite under inert atmosphere if possible
- Neutralize carefully with alcohol (e.g., isopropanol) under inert conditions
- Collect waste in approved hazardous container
- Exposure:
- Skin Contact: Flush with dry cloth first, then rinse with copious water; seek medical attention
- Eye Contact: Rinse immediately with water for at least 15 minutes; consult ophthalmologist
- Inhalation: Move to fresh air; administer oxygen if needed; seek medical help
8. Waste Disposal
- Method:
- Quench slowly with alcohol (e.g., isopropanol) under inert atmosphere
- Dispose of as hazardous organic waste per RCRA or local regulations
- Documentation:
- Maintain manifests and waste tracking records (EPA Form 8700-22 in U.S.)
- Use licensed hazardous waste disposal facilities
9. Regulatory Compliance
- OSHA (U.S.):
- Hazard Communication Standard (29 CFR 1910.1200) – SDS must be available
- PPE and training required
- EPA:
- Reportable quantities under CERCLA (1 lb for some organolithiums)
- REACH/CLP (EU):
- Pre-registered; classification as Carc. 1B, STOT RE 2, etc., may apply depending on formulation
- TSCA (U.S.): Listed chemical – no restrictions on import/manufacture
10. Safety Data Sheet (SDS) Requirements
Ensure SDS includes:
– Section 1: Identification (incl. UN number, emergency phone)
– Section 2: Hazard identification (H224, H250, H314, etc.)
– Section 7: Handling and storage
– Section 8: Exposure controls and PPE
– Section 13: Disposal considerations
– Section 14: Transport information (UN3405, Class 4.2/4.3)
11. Training & Documentation
- Personnel must be trained in:
- Pyrophoric handling techniques
- Inert atmosphere operations
- Emergency response (fire, spill, exposure)
- Maintain records of:
- Training logs
- Inventory tracking
- Incident reports
Disclaimer: This guide is for informational purposes only. Always consult the manufacturer’s SDS, local regulations, and institutional EHS policies before handling methyllithium.
Prepared in accordance with GHS Rev. 9, DOT 49 CFR, IATA DGR 2023, and IMDG Code 2022.
Conclusion on the Sourcing of Methyllithium
Methyllithium is a highly reactive and hazardous organolithium reagent commonly used in organic synthesis for carbon-carbon bond formation. Due to its pyrophoric nature—igniting spontaneously upon exposure to air—and its sensitivity to moisture, the sourcing, handling, and storage of methyllithium require strict safety protocols and specialized infrastructure.
When sourcing methyllithium, researchers and industrial users must rely on reputable chemical suppliers that provide the reagent in stabilized forms (typically in diethyl ether or other anhydrous solvents) and properly sealed, inert-atmosphere containers (e.g., septum-sealed bottles or cylinders under argon). Major chemical suppliers such as Sigma-Aldrich, TCI Chemicals, Alfa Aesar, and others offer various concentrations (e.g., 1.6 M or 3.0 M solutions) with detailed safety data sheets (SDS) and handling recommendations.
Alternative sourcing—such as on-site preparation—is technically feasible but generally discouraged outside of highly specialized laboratories due to the extreme hazards involved, including the use of metallic lithium and methyl halides, both of which present significant safety and regulatory challenges.
Ultimately, the safe and effective sourcing of methyllithium depends on:
- Supplier reliability and quality control;
- Proper packaging to maintain anhydrous and inert conditions;
- Compliance with transportation regulations for hazardous and flammable materials;
- Appropriate end-user infrastructure, including gloveboxes, Schlenk lines, and trained personnel.
Given these factors, purchasing from certified chemical suppliers remains the most practical, safe, and widely adopted method for obtaining methyllithium in academic and industrial settings. Safe handling and strict adherence to institutional safety guidelines are paramount to prevent accidents and ensure operational integrity.