The global market for medical devices aimed at improving blood circulation, particularly in the lower extremities, has seen robust growth driven by rising prevalence of circulatory disorders, an aging population, and increasing awareness of non-invasive therapeutic solutions. According to a report by Mordor Intelligence, the medical devices for vascular diseases market was valued at USD 35.8 billion in 2023 and is projected to grow at a CAGR of 6.9% from 2024 to 2029. This expansion is fueled by a surge in peripheral artery disease (PAD), diabetes-related complications, and venous insufficiency—conditions that often necessitate the use of leg blood circulation machines such as compression therapy devices, electrical muscle stimulators, and pneumatic compression systems.
Moreover, increasing adoption of home healthcare devices and technological advancements in wearable circulation aids have broadened the consumer base, encouraging new entrants and innovations. As demand rises, manufacturers are focusing on clinical efficacy, user comfort, and smart integration features like Bluetooth connectivity and mobile app monitoring. With North America and Europe leading in market share due to advanced healthcare infrastructure and high diagnosis rates, Asia-Pacific is emerging as the fastest-growing region due to expanding healthcare access and rising disposable incomes. In this evolving landscape, identifying the top manufacturers of leg blood circulation machines becomes crucial for healthcare providers, distributors, and consumers seeking reliable, evidence-based solutions.
Top 10 Leg Blood Circulation Machine Manufacturers 2026
(Ranked by Factory Capability & Trust Score)
#1 Power Plate
Domain Est. 2000
Website: powerplate.com
Key Highlights: Power Plate is the global leader in whole body vibration technology. Explore our products to help improve strength, flexibility, circulation & balance….
#2 Normatec 3 Legs: Complete Leg Recovery Suite
Domain Est. 2007
Website: hyperice.com
Key Highlights: In stock Rating 5.0 (334) Normatec’s patented precision pulse technology helps to increase circulation, revive muscles, and reduce swelling and has long been the choice of elite …
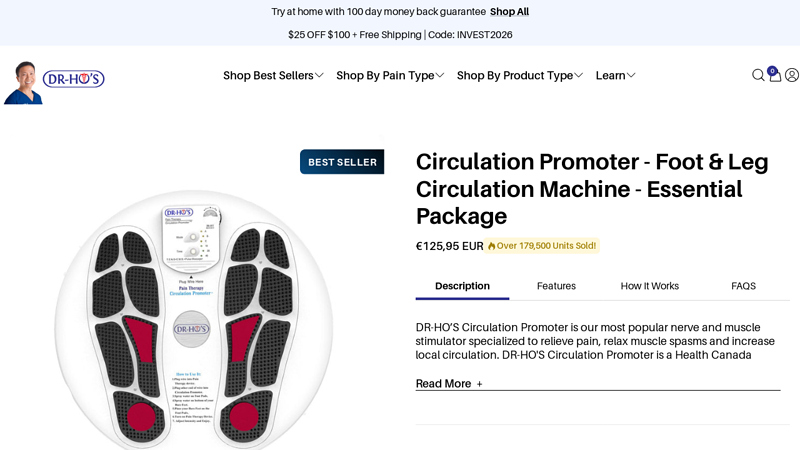
#3 DR-HO’S Circulation Promoter
Domain Est. 1999
Website: drhonow.com
Key Highlights: Rating 4.6 (1,242) · Free delivery over $144.14DR-HO’S Circulation Promoter is a doctor-developed, drug-free, medical-grade device designed to relieve muscle and joint pain whi…
#4 DR
Domain Est. 2006
Website: fsastore.com
Key Highlights: Can help to relieve symptomatic pain associated with: Foot and ankle pain, Temporary increase local blood circulation in healthy legs and feet, Muscle pain, ……
#5 Improve Blood Circulation
Domain Est. 2012
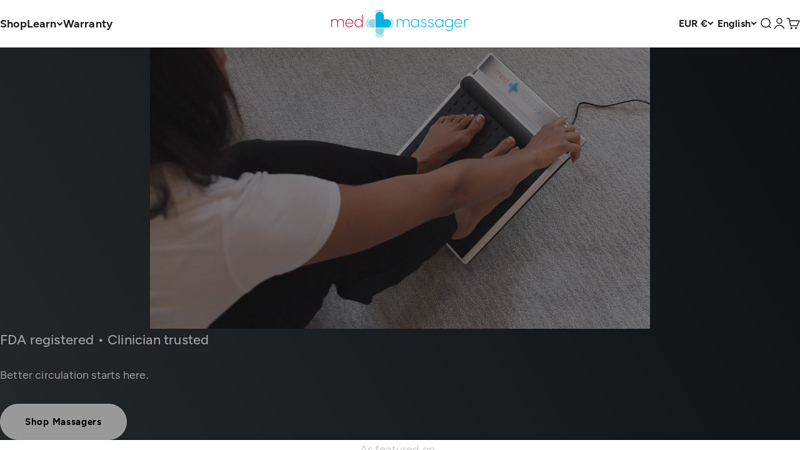
#6 MedMassager
Domain Est. 2012
Website: medmassager.com
Key Highlights: Stimulated nerve treatment boosts blood flow, relieving discomfort and chronic pain. Helps with RLS, plantar fasciitis, calf & knee strain, and more….
#7 air relax plus ar
Domain Est. 2016
Website: air-relax.com
Key Highlights: In stock Rating 5.0 (157) Air Relax Plus AR-3.0 is the FDA Cleared Medical Device that aim to reduce muscle fatigue and pain relief, remove excess fluid, and reduce swelling….
#8 Air
Domain Est. 2017
Website: reathlete.com
Key Highlights: Revitalize your legs with our adjustable compression system. Choose from three presets to ease pain, reduce swelling, and enhance circulation for refreshed ……
#9 Medcursor
Domain Est. 2017
Website: medcursor.com
Key Highlights: Free delivery 30-day returnsOur Cordless Foot and Leg Massager with Heat is particularly beneficial for individuals who sit for prolonged periods, those who stand for extended peri…
#10 Lumia Health
Domain Est. 2017
Website: lumiahealth.com
Key Highlights: Lumia is the only wearable that tracks blood flow to your head, so you can optimize your flow to feel better and think clearer….
Expert Sourcing Insights for Leg Blood Circulation Machine
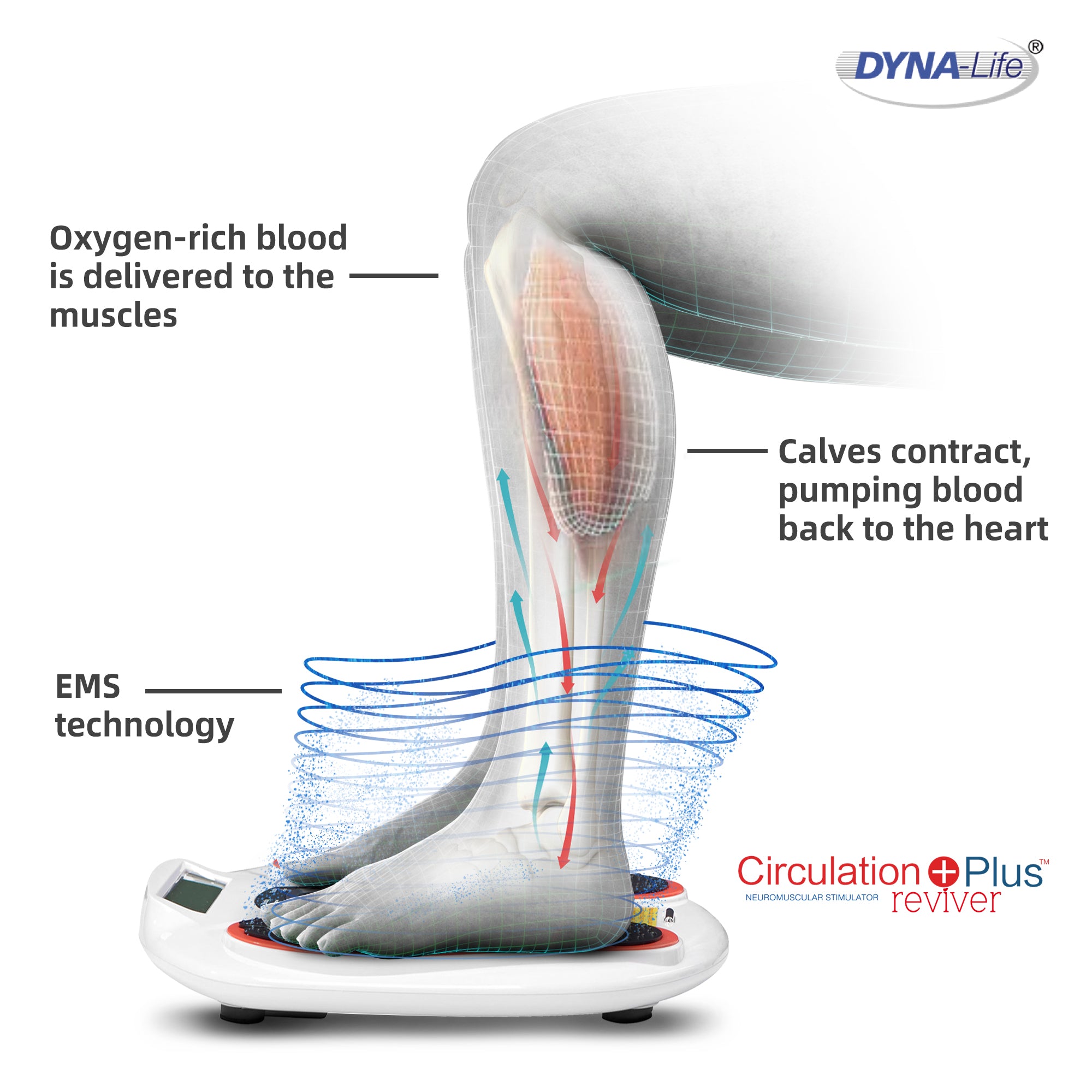
H2: 2026 Market Trends for Leg Blood Circulation Machines
The global market for leg blood circulation machines is projected to experience significant growth and transformation by 2026, driven by demographic shifts, rising prevalence of circulatory disorders, and advancements in medical technology. These devices—commonly referred to as dynamic leg exercisers, circulatory assist devices, or neuromuscular stimulators—are designed to improve blood flow in the lower limbs, primarily targeting individuals with venous insufficiency, diabetes-related complications, deep vein thrombosis (DVT) risk, and mobility limitations.
-
Aging Population and Chronic Disease Prevalence
A key driver of market expansion by 2026 is the growing elderly population worldwide, particularly in North America, Europe, and parts of Asia. As age increases, so does the risk of circulatory issues such as peripheral artery disease (PAD) and chronic venous insufficiency (CVI). Coupled with the rising incidence of diabetes and obesity—both of which impair circulation—demand for non-invasive, home-use leg circulation devices is expected to surge. -
Shift Toward Home-Based and Preventive Healthcare
The healthcare industry is increasingly embracing decentralized models, with patients preferring at-home treatments. By 2026, leg blood circulation machines are anticipated to become standard components of home healthcare regimens, especially for post-surgical recovery and long-term management of circulatory conditions. This trend is supported by the growing adoption of telehealth and remote patient monitoring, enabling clinicians to recommend and manage device usage remotely. -
Technological Advancements and Smart Integration
Innovation will play a pivotal role in shaping the 2026 market. Devices are expected to feature enhanced connectivity through Bluetooth and mobile applications, allowing users to track circulation metrics, set personalized therapy programs, and share data with healthcare providers. Integration with wearable health tech and AI-driven analytics will enable predictive care, adjusting therapy based on real-time physiological feedback. -
Expansion of Product Offerings and Portability
Manufacturers are anticipated to launch more compact, lightweight, and battery-operated models to improve user convenience and portability. This evolution will broaden market access beyond clinical settings into daily-use consumer environments, including workplaces and travel scenarios—especially for individuals at risk of DVT during long-haul flights. -
Regulatory Support and Reimbursement Improvements
Regulatory bodies in key markets such as the U.S. (FDA), EU (CE marking), and Japan (PMDA) are streamlining approval pathways for Class II medical devices like circulation machines. By 2026, increased insurance coverage and reimbursement options—particularly in Medicare and private health plans—are expected to lower patient out-of-pocket costs, accelerating adoption. -
Regional Market Growth
While North America currently dominates the market due to high healthcare spending and awareness, the Asia-Pacific region is projected to witness the fastest growth by 2026. Countries like China, India, and South Korea are investing heavily in healthcare infrastructure and chronic disease management, creating fertile ground for market entry and expansion. -
Competitive Landscape and Strategic Partnerships
The market will likely see increased consolidation, with major medical device companies acquiring niche innovators or forming partnerships with digital health platforms. Emphasis will be placed on user-centric design, clinical validation, and direct-to-consumer marketing strategies to capture a larger share of the wellness and medical device markets.
In conclusion, the leg blood circulation machine market in 2026 will be characterized by technological sophistication, expanded accessibility, and integration into broader digital health ecosystems. With growing awareness and medical validation, these devices are poised to transition from niche therapeutic tools to mainstream solutions for vascular health management.

Common Pitfalls When Sourcing Leg Blood Circulation Machines (Quality and Intellectual Property)
Sourcing leg blood circulation machines—often pneumatic compression devices designed to improve venous return and reduce swelling—can be complex, especially when balancing cost, quality, and legal compliance. Overlooking key risks related to product quality and intellectual property (IP) can lead to regulatory issues, safety concerns, and financial losses. Here are the most common pitfalls to avoid:
Poor Manufacturing Quality and Non-Compliant Materials
Many suppliers, particularly in low-cost manufacturing regions, may use substandard materials or cut corners in production. This includes using inferior plastics, unreliable pumps, or poorly calibrated pressure sensors, which can compromise device effectiveness and patient safety. Devices may not meet biocompatibility standards or may fail prematurely under regular use.
Tip: Always request material certifications (e.g., ISO 10993 for biocompatibility) and conduct third-party quality audits of the factory.
Lack of Regulatory Certification (e.g., FDA, CE, ISO 13485)
A major red flag is sourcing from manufacturers who cannot provide proof of regulatory compliance. Leg circulation devices are typically classified as medical devices and require certifications like FDA 510(k), CE Marking under MDR, or compliance with ISO 13485 for quality management systems. Devices without these may be illegal to sell in target markets.
Tip: Verify the manufacturer’s regulatory dossier and ensure the specific model you’re sourcing is listed under valid certifications.
Inadequate Performance Testing and Clinical Validation
Some suppliers claim their devices improve circulation but provide no clinical data or performance validation. Without proper testing (e.g., pressure consistency, cycle durability, or patient outcome studies), you risk marketing an ineffective or even harmful product.
Tip: Request test reports, including pressure delivery accuracy and cycle life testing. Consider independent lab validation if clinical data is unavailable.
Risk of IP Infringement from Copycat Designs
Many leg circulation machines on the market, particularly from generic manufacturers, closely mimic patented designs from established brands (e.g., compression waveform patterns, control unit interfaces, or inflatable sleeve configurations). Sourcing such devices may expose your company to intellectual property litigation, including patent, design, or utility model infringement.
Tip: Conduct an IP landscape analysis before finalizing a supplier. Work with a patent attorney to review key design and functional elements of the device.
Unclear or Missing IP Ownership in Manufacturing Agreements
Even if a device appears original, suppliers may not fully transfer IP rights, or may have shared designs with multiple clients. This creates risks of parallel sales, reverse engineering by competitors, or inability to customize the product without legal complications.
Tip: Ensure your manufacturing contract explicitly states that tooling, custom designs, and any improvements are your exclusive property.
Counterfeit or Refurbished Units Marketed as New
Some suppliers may pass off refurbished, used, or counterfeit devices as new. This is particularly common in online B2B marketplaces. These units may have degraded performance, worn seals, or outdated software.
Tip: Use trusted sourcing channels, conduct on-site inspections, and verify serial numbers against manufacturer databases when possible.
Inconsistent Software/Firmware and Lack of Updates
Modern leg circulation devices often include digital controls and firmware. Sourcing from manufacturers with poor software development practices can result in bugs, security vulnerabilities, or inability to update devices post-launch—posing safety and compliance risks.
Tip: Evaluate the supplier’s software development lifecycle (SDLC) and ensure they follow IEC 62304 standards for medical device software.
By proactively addressing these quality and IP-related pitfalls, businesses can reduce risk, ensure regulatory compliance, and bring safe, reliable, and legally sound leg circulation devices to market.

Logistics & Compliance Guide for Leg Blood Circulation Machine
Product Classification and Regulatory Requirements
The Leg Blood Circulation Machine is typically classified as a medical device, often falling under Class II (in the U.S. FDA system) or Class IIa/IIb (under EU MDR), depending on its intended use, mechanism (e.g., electrical muscle stimulation, pneumatic compression), and claimed therapeutic benefits. It is essential to confirm the exact classification in all target markets. In the U.S., the device may require 510(k) premarket notification. In the European Union, CE marking under the Medical Device Regulation (MDR) 2017/745 is mandatory, requiring technical documentation, risk management (ISO 14971), and quality management system (ISO 13485) compliance.
Intended Use and Labeling Compliance
Clear and accurate labeling is critical. The device labeling must include:
– Manufacturer name and address
– Device name and model number
– Intended use statement (e.g., “For improving blood circulation in the lower limbs through sequential compression”)
– Contraindications, warnings, and precautions
– Instructions for use (IFU) in the local language(s)
– Unique Device Identifier (UDI) in regions requiring it (e.g., U.S., EU, UK)
Ensure all promotional materials and user documentation align with regulatory clearances and avoid unsubstantiated medical claims.
Quality Management System (QMS)
Manufacturers and distributors must implement a certified Quality Management System compliant with ISO 13485. This includes documented procedures for design control, supplier management, production, corrective and preventive actions (CAPA), and post-market surveillance. Regular internal audits and management reviews are required to maintain compliance.
Import/Export Documentation and Customs Clearance
Prepare the following documentation for international shipments:
– Commercial invoice with HS code (typically 9018.90 for medical electrotherapy equipment)
– Packing list
– Certificate of Conformity (CE, FDA, etc.)
– Certificate of Free Sale (if required by destination country)
– Bill of lading or air waybill
– U.S. FDA Prior Notice (for U.S. imports)
Ensure proper product description and harmonized system (HS) code classification to avoid customs delays or misclassification.
Transportation and Storage Conditions
The device should be shipped in protective packaging to prevent damage during transit. While not generally temperature-sensitive, avoid extreme heat, cold, or humidity. Store in a dry, clean environment with controlled temperature (10°C to 30°C recommended). Follow IATA regulations if shipping lithium batteries (if applicable).
Post-Market Surveillance and Vigilance Reporting
Establish a system for monitoring device performance in the field. Report adverse events and field safety corrective actions (FSCAs) to relevant authorities within mandated timelines (e.g., 10–30 days in EU, 30 days in U.S. per FDA MedWatch). Maintain a U.S. FDA Medical Device Reporting (MDR) file and EU Incident Reporting system as required.
Country-Specific Compliance Considerations
- United States: FDA registration and listing, 510(k) clearance, UDI compliance, and state-level sales tax and distribution licensing may apply.
- European Union: CE marking under MDR, appointment of an EU Authorized Representative, and EUDAMED registration.
- United Kingdom: UKCA marking (or CE until 2027), UK Responsible Person, and MHRA registration.
- Canada: License under Health Canada’s Medical Devices Regulations, with a Canadian Medical Device License (MDL).
- Australia: Inclusion in the Australian Register of Therapeutic Goods (ARTG).
Environmental and Disposal Compliance
Ensure compliance with WEEE (Waste Electrical and Electronic Equipment) directives in applicable regions. Provide end-of-life disposal instructions to users and support take-back programs where required.
Training and Technical Support
Provide training materials and accessible technical support for healthcare providers and end users. Maintain records of customer inquiries and complaints as part of post-market surveillance obligations.
Conclusion for Sourcing a Leg Blood Circulation Machine:
Sourcing a leg blood circulation machine is a crucial decision that can significantly impact patient outcomes, especially for individuals suffering from poor circulation, venous insufficiency, diabetes-related complications, or those recovering from surgery. After evaluating various factors such as device efficacy, safety certifications (e.g., FDA, CE), ease of use, portability, cost, and patient-specific needs, it becomes clear that selecting the right device requires a balanced approach between quality, reliability, and value.
Investing in a clinically proven circulation device—such as those utilizing Sequential Gradient Compression (SGC) technology—not only enhances blood flow and reduces the risk of deep vein thrombosis (DVT), but also contributes to faster rehabilitation and improved quality of life. Additionally, sourcing from reputable manufacturers with strong customer support and warranties ensures long-term usability and peace of mind.
In conclusion, careful consideration of medical requirements, expert recommendations, and product specifications will lead to an informed sourcing decision. Prioritizing patient safety and therapeutic effectiveness ensures that the chosen leg blood circulation machine delivers optimal health benefits and represents a worthwhile investment in long-term vascular care.