The global lactase enzyme market is experiencing steady expansion, driven by rising lactose intolerance awareness and growing demand for lactose-free dairy products. According to Mordor Intelligence, the lactase enzyme market was valued at approximately USD 70 million in 2023 and is projected to grow at a CAGR of over 6.5% from 2024 to 2029. This growth is fueled by increased consumer preference for functional foods, advances in enzyme immobilization technologies, and expanding applications in the dairy and food processing industries. As the need for efficient lactose hydrolysis solutions intensifies, manufacturers are scaling production and enhancing enzyme efficacy. In this evolving landscape, nine key players have emerged as leading lactase powder suppliers, combining biotechnological innovation, regulatory compliance, and global distribution networks to meet growing market demands.
Top 9 Lactase Powder Manufacturers 2026
(Ranked by Factory Capability & Trust Score)
#1 Lactose & Organic Lactose Powders
Domain Est. 1997
Website: themilkywhey.com
Key Highlights: The Milky Whey has edible lactose available for all of your lactose needs including: ice cream, skim milk, condensed milk, dry soups, coffee creamers, ……
#2 Lactase Powder Lactozym Manufacturers and Suppliers
Domain Est. 2012
Website: fengchengroup.com
Key Highlights: Fengchen Group is a leading China Lactase Powder Lactozym supplier and manufacturer. We specialize in wholesale and bulk amounts….
#3 Lactose-Free Dairy Products
Domain Est. 1996
Website: lactaid.com
Key Highlights: We’ve got a line of lactose-free dairy products so you can enjoy all of the goodness of real milk and dairy, just without the discomfort!…
#4 Lactase
Domain Est. 2003
Website: mpbio.com
Key Highlights: Its primary commercial use is to break down lactose in milk to make it suitable for people with lactose intolerance. Usage Statement. Unless specified otherwise ……
#5 Lactose India Limited
Domain Est. 2003
Website: lactoseindialimited.com
Key Highlights: We are an exclusive site for manufacturing Dry Granulated, Film and Lacquer coated Tablets for Sanofi India with over 15 years of experience. Our installed ……
#6 Lactose Powder
Domain Est. 2007
Website: lactalisingredients.com
Key Highlights: Lactose powder is available either in white or standard colour. LACTALIS Ingredients offers a wide range of lactose to best meet customers’ expectations….
#7 Lactase
Domain Est. 2009
#8 Lactase Enzyme (3000 ALU) Powder
Domain Est. 2011
#9 Lactase 65000 units/gm enzyme
Domain Est. 2016
Website: cambridgecommodities.com
Key Highlights: Derived from a natural source, our Lactase enzyme powder is meticulously formulated to support optimal digestion of lactose, a challenging component found in ……
Expert Sourcing Insights for Lactase Powder

H2: 2026 Market Trends for Lactase Powder
The global lactase powder market is poised for significant growth and transformation by 2026, driven by evolving consumer preferences, technological advancements, and expanding applications across food, dietary supplements, and pharmaceutical sectors. Key trends shaping the market include rising lactose intolerance awareness, clean-label product demand, innovations in enzyme production, and regional market dynamics.
-
Increased Prevalence of Lactose Intolerance
Growing awareness and diagnosis of lactose intolerance worldwide are primary drivers of lactase powder demand. With an estimated 65–70% of the global population exhibiting some degree of lactose malabsorption, consumers are increasingly seeking lactose-free or reduced-lactose dairy products. This shift is fueling demand for lactase enzymes used in the pre-treatment of milk and dairy-based products, directly boosting the lactase powder market. -
Expansion in Functional Foods and Dairy Alternatives
The functional food and beverage sector is integrating lactase powder to develop digestible dairy products, including lactose-free milk, yogurt, and ice cream. Additionally, plant-based dairy alternatives often incorporate lactase to mimic the nutritional profile and taste of traditional dairy, enhancing product appeal. This trend is particularly strong in North America and Europe, where health-conscious consumers prioritize digestive wellness. -
Clean Label and Natural Ingredient Demand
Consumers are favoring transparent, natural, and non-GMO ingredients. In response, manufacturers are investing in microbial-derived lactase (e.g., from Aspergillus oryzae or Kluyveromyces lactis) that meet clean-label standards. This shift is encouraging biotech firms to improve fermentation processes and develop high-purity, allergen-free lactase powders suitable for clean-label applications. -
Technological Advancements in Enzyme Efficiency
By 2026, innovations in enzyme immobilization and thermo-stable lactase variants are expected to enhance processing efficiency in industrial dairy applications. These advancements reduce production costs and improve lactose hydrolysis efficacy, making lactase powder more attractive to large-scale dairy processors. Research into genetically optimized strains continues to improve yield and specificity. -
Growth in Dietary Supplements
The nutraceutical sector is increasingly incorporating lactase powder into over-the-counter digestive enzyme supplements. With rising self-management of digestive health, especially among aging populations and frequent travelers, demand for lactase supplements is growing in regions like Asia-Pacific and Latin America. -
Regional Market Expansion
While North America and Europe remain dominant due to high health awareness and regulatory support, the Asia-Pacific region is expected to witness the fastest growth. Urbanization, rising disposable incomes, and increased healthcare access in countries like China and India are expanding the consumer base for lactose-free products and related enzymes. -
Sustainability and Green Manufacturing
Environmental concerns are pushing manufacturers toward sustainable production methods. Biotechnological processes with lower energy consumption and reduced waste are becoming a competitive advantage. Companies emphasizing eco-friendly manufacturing are likely to gain market share as ESG (Environmental, Social, and Governance) criteria influence purchasing decisions.
In conclusion, the 2026 lactase powder market will be shaped by health-driven consumer behavior, technological innovation, and regional diversification. Stakeholders who invest in high-efficiency, natural, and sustainably produced lactase powders are well-positioned to capitalize on these emerging trends.
Common Pitfalls When Sourcing Lactase Powder (Quality & Intellectual Property)
Sourcing lactase enzyme powder for food, dietary supplements, or industrial applications involves navigating significant challenges related to quality assurance and intellectual property (IP) protection. Overlooking these areas can lead to product failure, regulatory non-compliance, financial losses, and legal disputes.
Quality-Related Pitfalls
1. Inadequate Enzyme Activity Verification
– Relying solely on supplier-provided Certificates of Analysis (CoA) without independent verification.
– Failing to test for actual lactase activity (measured in FCC ALU/g or LacU/g) under real-use conditions (e.g., at refrigeration temperatures for dairy applications).
– Not accounting for activity loss during storage or processing (heat, pH extremes).
2. Poor Purity and Contaminant Control
– Accepting products with high levels of residual proteins, carbohydrates, or microbial contaminants (e.g., endotoxins, heavy metals).
– Overlooking allergen cross-contamination risks, especially if manufactured in shared facilities.
– Insufficient screening for excipients or anti-caking agents that may affect formulation or labeling.
3. Inconsistent Batch-to-Batch Performance
– Selecting suppliers without robust process controls, leading to variability in enzyme kinetics, pH/temperature profiles, or shelf life.
– Not implementing a supplier qualification program with ongoing audits and performance monitoring.
4. Misrepresentation of Microbial Source and Safety
– Assuming all microbial-derived lactase (e.g., from Aspergillus oryzae, Kluyveromyces lactis) is equally safe without reviewing GRAS (Generally Recognized As Safe) status or regulatory dossiers.
– Failing to verify that the strain used is non-pathogenic and free from mycotoxin production.
Intellectual Property-Related Pitfalls
1. Unlicensed Use of Patented Enzyme Strains or Processes
– Sourcing lactase produced via patented fermentation or immobilization methods without proper licensing.
– Using high-efficiency or thermostable variants protected by IP, especially in competitive markets like dairy processing.
2. Infringement of Formulation or Application Patents
– Incorporating lactase into a final product (e.g., lactose-free milk, digestive supplements) in a manner that infringes on method-of-use patents held by competitors.
– Overlooking regional IP differences—patents valid in one country may not apply in another.
3. Lack of Freedom-to-Operate (FTO) Analysis
– Proceeding with supplier selection or product development without conducting an FTO search to identify third-party patents covering the enzyme, its use, or delivery system.
– Assuming generic availability of lactase without verifying the IP landscape, especially for engineered or optimized variants.
4. Ambiguous IP Ownership in Custom Development
– Engaging contract manufacturers or developers without clear contractual terms on IP ownership of process improvements or novel formulations.
– Risk of losing rights to proprietary blends or delivery systems developed jointly.
Mitigation Strategies
- Require comprehensive CoAs including activity, purity, microbiological safety, and allergen testing.
- Conduct independent lab testing and stability studies.
- Perform due diligence on supplier manufacturing practices (GMP, ISO certifications).
- Consult IP counsel to conduct FTO analyses and secure necessary licenses.
- Include clear IP clauses in supplier agreements, especially for custom or co-developed products.
Avoiding these pitfalls ensures reliable product performance, regulatory compliance, and protection against legal exposure in the competitive enzyme marketplace.

Logistics & Compliance Guide for Lactase Powder
Product Overview
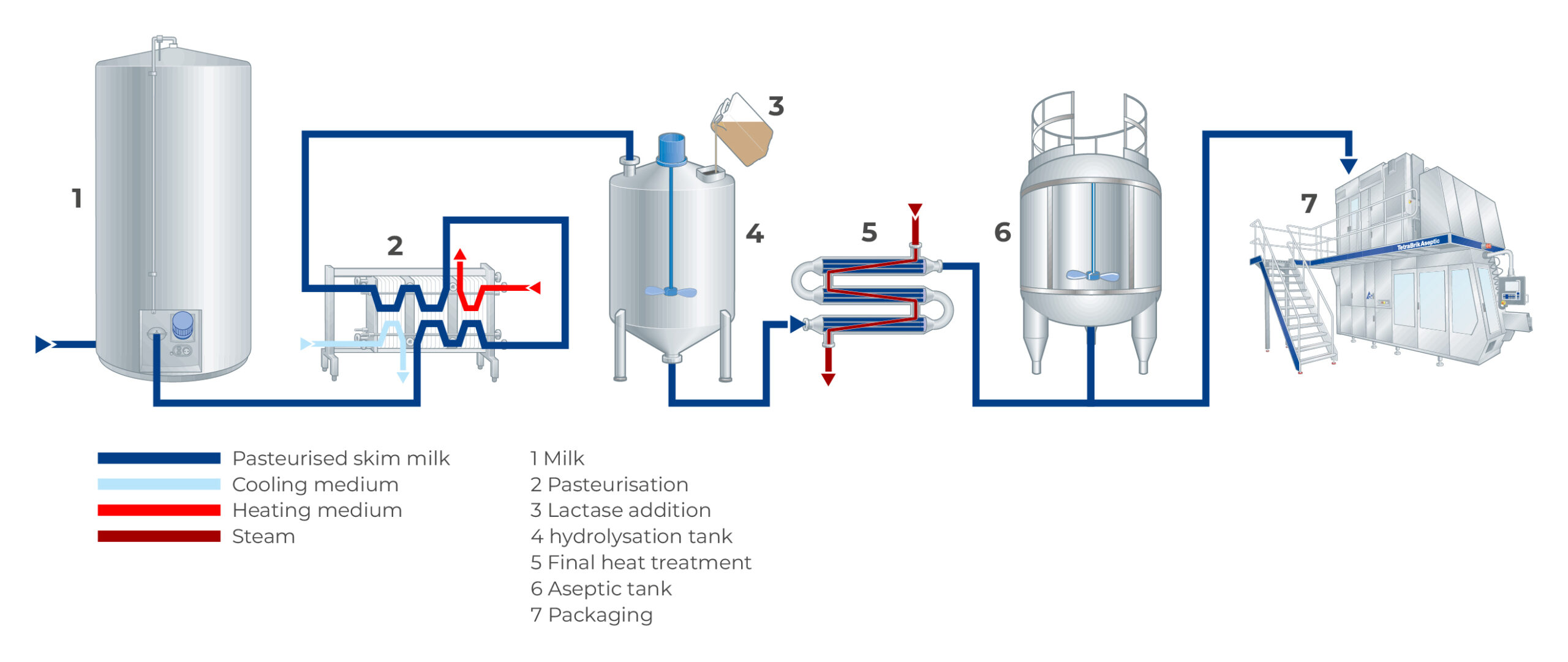
Lactase powder is an enzymatic food additive used to break down lactose in dairy products, aiding in lactose intolerance management. It is typically derived from microbial sources (e.g., Aspergillus oryzae or Kluyveromyces lactis) and supplied as a fine, free-flowing powder. Proper logistics and compliance handling are essential to maintain enzyme activity, ensure safety, and meet regulatory standards.
Regulatory Classification & Approvals
Lactase powder is classified as a food enzyme and/or processing aid, depending on the jurisdiction. Compliance with regional and international regulations is required:
- United States (FDA): Generally Recognized as Safe (GRAS) under 21 CFR 184.1415 when used in accordance with Good Manufacturing Practices (GMP). Must comply with FDA food additive regulations if making specific health claims.
- European Union (EFSA): Approved as a food enzyme under Regulation (EC) No 1332/2008. Listed in the Union Catalogue of Approved Food Enzymes (Commission Implementing Regulation (EU) No 872/2012).
- Codex Alimentarius: Recognized in the General Standard for Food Additives (GSFA) under enzyme preparations.
- Other Regions: Approval may be required from local food safety authorities (e.g., Health Canada, FSANZ in Australia/New Zealand). Always verify country-specific import requirements.
Ensure supplier documentation includes a Certificate of Compliance (CoC) and, where applicable, a Novel Food or Enzyme dossier.
Labeling Requirements
Labeling must comply with food safety and consumer protection regulations in the target market:
- Common Name: Lactase (or β-galactosidase)
- Source Organism: e.g., Aspergillus oryzae
- Activity Level: Expressed in enzyme units (e.g., ALU/g – Acid Lactase Units)
- Net Weight
- Batch/Lot Number
- Best Before Date
- Storage Conditions
- Manufacturer/Importer Information
- Allergen Statement: If produced in a facility handling allergens (e.g., gluten, soy, milk traces)
- Usage Instructions: Recommended dosage and application
Labels must be in the official language(s) of the destination country.
Packaging & Handling
- Primary Packaging: Use moisture-resistant, sealed containers (e.g., double-lined polyethylene bags, aluminum foil pouches, or HDPE bottles) to protect from humidity and contamination.
- Secondary Packaging: Ship in sturdy, undamaged cartons with cushioning material to prevent damage during transit.
- Handling Precautions: Use clean, dry equipment. Avoid cross-contamination with allergens or incompatible substances. Minimize dust generation; use PPE (gloves, mask) when handling bulk powder.
Storage Conditions
- Temperature: Store in a cool, dry place. Recommended range: 2–25°C (36–77°F). Avoid exposure to high heat.
- Humidity: Keep relative humidity below 60% to prevent clumping and loss of enzyme activity.
- Light: Protect from direct sunlight; store in opaque or light-resistant containers.
- Shelf Life: Typically 12–24 months when stored properly. Monitor activity periodically if stored long-term.
Transportation & Shipping
- Mode of Transport: Suitable for air, sea, and land freight. Ensure compliance with IATA (air) or IMDG (sea) guidelines if applicable (though lactase powder is generally non-hazardous).
- Temperature Control: Use ambient or temperature-controlled transport if climate conditions exceed storage limits.
- Documentation: Include commercial invoice, packing list, CoA (Certificate of Analysis), CoC, and any required import permits or sanitary certificates.
- Customs Clearance: Declare under correct HS Code (e.g., 3507.90 for enzyme preparations, non-specific). Confirm tariff and import duty with local customs.
Quality & Safety Documentation
- Certificate of Analysis (CoA): Provided by manufacturer/supplier, including:
- Enzyme activity (ALU/g or similar)
- Microbiological specifications (total plate count, yeast/mold, pathogens)
- Heavy metals (e.g., lead, arsenic, mercury)
- Moisture content
- Residual solvents (if applicable)
- Allergen Declaration: Confirm absence of major allergens or declare potential cross-contact.
- GMP/HACCP Certification: Ensure manufacturing facility is certified to relevant food safety standards.
Import/Export Compliance
- Import Permits: Some countries require prior approval for enzyme imports (e.g., China, India). Check with local food safety authority.
- Phytosanitary/Sanitary Certificates: May be required depending on country of origin and destination.
- Customs Classification: Accurately declare product as a food enzyme or additive. Misclassification may lead to delays or penalties.
Risk Management
- Contamination Risk: Prevent exposure to moisture, strong odors, and toxic substances.
- Dust Explosion Risk: Although low, lactase powder is combustible in fine dust form. Follow standard dust control practices in bulk handling.
- Allergenicity: While lactase itself is not a major allergen, trace residues from production organisms or processing aids may pose risks. Label accordingly.
Disposal & Environmental Considerations
Dispose of expired or contaminated lactase powder in accordance with local waste regulations. It is biodegradable but should not be released in large quantities into waterways without treatment.
Summary
Proper logistics and compliance management for lactase powder ensures product efficacy, regulatory adherence, and consumer safety. Key focus areas include correct classification, compliant labeling, controlled storage, and thorough documentation. Always verify regulatory requirements in both exporting and importing countries and maintain traceability through batch records and certifications.
Conclusion for Sourcing Lactase Powder:
Sourcing lactase powder requires a comprehensive evaluation of several key factors including product quality, supplier reliability, regulatory compliance, cost-effectiveness, and technical support. Ensuring the lactase enzyme meets food-grade or pharmaceutical-grade standards—depending on the intended application—is critical for safety, efficacy, and market acceptance. Working with reputable suppliers who provide transparent documentation, such as Certificates of Analysis (CoA), allergen statements, and compliance with international standards (e.g., FDA, EU regulations, HALAL/KOSHER certifications), minimizes risks and ensures consistency in production.
Additionally, considering enzyme activity units (e.g., FCC lactase units), formulation (powder vs. granulated), and storage requirements will influence the suitability for specific applications such as dairy processing, dietary supplements, or lactose-free product development. Building long-term relationships with suppliers who offer scalability, responsive customer service, and innovation support can provide a strategic advantage.
Ultimately, a well-structured sourcing strategy for lactase powder—focused on quality, compliance, and partnership—will support product integrity, consumer satisfaction, and regulatory success in the competitive food and nutraceutical industries.