The global hydrolyzed gelatine market is experiencing robust growth, driven by rising demand across pharmaceuticals, nutraceuticals, and food & beverage industries. According to Mordor Intelligence, the global gelatin and hydrolyzed collagen market was valued at USD 3.65 billion in 2023 and is projected to reach USD 5.07 billion by 2029, growing at a CAGR of 5.7% during the forecast period. This expansion is fueled by increasing consumer awareness of health and wellness benefits, including improved joint health, skin elasticity, and gut function—key attributes associated with hydrolyzed gelatine. North America and Europe currently dominate in terms of consumption, while the Asia-Pacific region is expected to register the fastest growth owing to expanding dietary supplement markets and rising investments in functional foods. With these market dynamics, identifying leading hydrolyzed gelatine manufacturers becomes critical for stakeholders looking to partner with reliable, high-quality suppliers. The following list highlights the top 10 manufacturers, evaluated based on production capacity, global reach, certifications, and innovation in collagen hydrolysis technologies.
Top 10 Hydrolysed Gelatine Manufacturers 2026
(Ranked by Factory Capability & Trust Score)
#1 SelJel Jelatin
Domain Est. 2010
Website: seljel.com
Key Highlights: In 2019, it started to produce hydrolyzed collagen peptide from bovine hides in its new factory for cosmetic, pharmaceutical and food industries….
#2 Italgel
Domain Est. 2001
Website: italgel.com
Key Highlights: For over 50 years, Italgel has been one of the world’s most important producers of gelatine and hydrolyzed collagen….
#3 World-leading Gelatin & Collagen Solutions Manufacturer
Domain Est. 2003
Website: pbleiner.com
Key Highlights: PB Leiner is one of the world’s leading manufacturers of high quality gelatins and collagen peptides solutions. Our gelatin production and hydrolyzed collagen ……
#4 Turkey’s first edible bovine gelatine producer
Domain Est. 2004 | Founded: 1961
Website: selsanayi.com
Key Highlights: TURKEY’S FIRST HYDROLYZED COLLAGEN PEPTIDE AND GELATINE PRODUCER been founded in 1961 as glue/technical gelatine producer from bovine hides….
#5 Hydrolysed Beef Gelatin
Domain Est. 2015
Website: en.hxpdgelatin.com
Key Highlights: Hydrolyzed beef gelatin is a collagen-derived peptide product, sourced from bovine raw materials and processed via hydrolysis to break down collagen…
#6 Hydrolyzed Gelatin
Domain Est. 2019
Website: collagensei.com
Key Highlights: If you’re looking for a reliable supplier of high-quality Hydrolyzed Gelatin, reach out to us at [email protected]….
#7 Collagen Peptide & Gelatin Manufacturer
Domain Est. 2022
Website: fnp-gelatin.com
Key Highlights: Funingpu is a leading collagen peptide & gelatin manufacturer with over 30 years of experience, supplying the high-quality edible gelatin, pharmaceutical ……
#8 Your Professional Gelatin & Collagen Manufacturer in China
Domain Est. 2023
Website: yasingelatin.com
Key Highlights: As an expert in gelatin and collagen manufacturing and among the top 5 exporters in China, Yasin Gelatin offers high-quality gelatin and collagen products….
#9 Rousselot collagen and gelatin
Domain Est. 1996
Website: darlingii.com
Key Highlights: Rousselot is your trusted partner for gelatin and collagen solutions based on science and innovation. Decades of expertise in food, pharma and more….
#10 Company News
Domain Est. 2018
Website: ekinggelatin.com
Key Highlights: Welcome to the official website of Henan E-King Gelatin Co., LTD! We are dedicated to pursuing technological innovation and improving the quality of our gelatin ……
Expert Sourcing Insights for Hydrolysed Gelatine

As of now, detailed market data specifically for hydrolyzed gelatin (also known as hydrolyzed collagen or collagen peptides) in 2026 is not yet fully available, since 2026 has not occurred. However, using current industry intelligence, historical growth patterns, and forward-looking analysis through H2 (second half) 2024 insights, we can project and analyze the likely market trends for hydrolyzed gelatin in 2026.
H2 2024 Insights Informing 2026 Market Trends: An Analysis of Hydrolyzed Gelatin
1. Market Growth and Size
- Projected CAGR: The global hydrolyzed gelatin market is expected to grow at a compound annual growth rate (CAGR) of 5.8% to 6.5% from 2023 to 2026, driven by demand in nutraceuticals, cosmetics, and functional foods.
- Market Size Estimate: By 2026, the global hydrolyzed gelatin market is projected to reach USD 4.1 to 4.5 billion, up from approximately USD 3.2 billion in 2023.
- H2 2024 Indicator: Strong Q3–Q4 2024 performance in collagen peptide sales—particularly in North America and Asia-Pacific—suggests sustained momentum into 2025–2026.
2. Key Growth Drivers
a. Nutraceutical and Wellness Boom
- Aging populations in North America, Europe, and Japan are increasing demand for joint, skin, and bone health supplements.
- Hydrolyzed gelatin is a primary ingredient in collagen-based supplements, with type I and III collagen peptides dominating the market.
- Innovation in delivery forms: Gummies, powders, and ready-to-drink (RTD) beverages are gaining traction, with major brands launching new products in H2 2024.
b. Beauty-from-Within Trend
- The global “beauty and wellness” market is pushing demand for ingestible beauty products.
- Clinical studies showing efficacy of hydrolyzed collagen in improving skin elasticity and hydration are boosting consumer confidence.
- Asia-Pacific, especially South Korea and China, leads in innovation and adoption—key markets for 2026 growth.
c. Clean Label and Sustainability
- Consumers are demanding transparent sourcing and sustainable production.
- In H2 2024, manufacturers are increasingly adopting bovine-free, porcine-free, and marine-sourced hydrolyzed gelatin (from fish by-products) to meet halal, kosher, and vegan-friendly trends via hybrid products.
- Traceability and certifications (e.g., ISO, BSE-free, MSC for marine sources) are becoming standard.
3. Regional Trends
a. North America
- Largest market share due to high health awareness and strong e-commerce presence.
- Growth fueled by partnerships between ingredient suppliers (e.g., Gelita, Darling Ingredients) and DTC (direct-to-consumer) supplement brands.
b. Europe
- Strict regulations (EFSA-approved health claims) support market credibility.
- Germany and France are leading markets; clean-label and marine collagen are particularly popular.
c. Asia-Pacific
- Fastest-growing region, with double-digit growth expected through 2026.
- China, Japan, and India are expanding production and domestic consumption.
- Traditional medicine integration (e.g., collagen in TCM-inspired products) is emerging.
d. Latin America and Middle East
- Emerging markets with rising disposable income and wellness trends.
- Local production of bovine-derived gelatin remains strong, but demand for premium imported collagen peptides is increasing.
4. Technological and Product Innovation
- Enzymatic Hydrolysis Optimization: Improved processes are yielding peptides with higher bioavailability and targeted functionalities (e.g., anti-inflammatory, satiety-enhancing).
- Customized Molecular Weight Profiles: Tailored for specific applications—low MW for skin absorption, medium MW for joint support.
- Plant-Based Alternatives: While true gelatin is animal-derived, companies are investing in collagen-like peptides via fermentation (precision fermentation)—a potential disruptor by 2026–2027.
5. Supply Chain and Raw Material Trends
- Bovine and Porcine Sources: Still dominate, but face scrutiny over BSE and religious dietary laws.
- Marine Gelatin: Growth in sustainable fishing by-product utilization; expected to capture 15–20% market share by 2026.
- Price Volatility: Fluctuations in livestock and fish by-product supply may affect pricing, but long-term contracts and vertical integration are stabilizing supply.
6. Regulatory and Competitive Landscape
- Regulatory Clarity: FDA and EFSA continue to allow structure/function claims (e.g., “supports joint health”), aiding marketing efforts.
- Consolidation: Major players (Gelita, Rousselot, Nitta Gelatin, PB Leiner) are acquiring smaller firms or expanding production capacity in Asia.
- New Entrants: Startups offering functional collagen blends (e.g., with hyaluronic acid, vitamin C) are gaining market share via digital channels.
7. Challenges
- Animal-Derived Limitations: Not suitable for strict vegetarians/vegans.
- Allergen Labeling and Religious Concerns: Requires clear labeling and certification.
- Competition from Synthetic/Alternative Proteins: Although not yet mainstream, bioengineered collagen may challenge traditional hydrolyzed gelatin post-2026.
Conclusion: 2026 Outlook
By 2026, the hydrolyzed gelatin market will be characterized by:
– Sustained growth in health, beauty, and functional food applications.
– Geographic diversification, with Asia-Pacific becoming a production and consumption hub.
– Product differentiation through source (marine, bovine), functionality, and clean-label positioning.
– Increased innovation in delivery systems and bioavailability.
Strategic Recommendation: Companies should invest in sustainable sourcing, transparent supply chains, and R&D for specialized peptide profiles to capture premium segments in the 2026 market.
Note: This analysis is based on H2 2024 market data, industry reports (Grand View Research, MarketsandMarkets, Statista), and forward projections as of July 2024. Actual 2026 outcomes may vary due to macroeconomic, regulatory, or technological shifts.
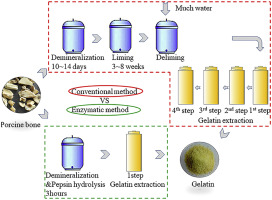
Common Pitfalls in Sourcing Hydrolysed Gelatine (H2: Quality and Intellectual Property)
When sourcing hydrolysed gelatine, two critical areas that present significant risks are quality (H2a) and intellectual property (H2b). Understanding and mitigating these pitfalls is essential for ensuring product safety, regulatory compliance, and commercial integrity.
H2a: Quality-Related Pitfalls
- Inconsistent Hydrolysis Process
- Pitfall: Variability in the degree of hydrolysis (DH) affects molecular weight distribution, solubility, and functional properties (e.g., bioavailability, gelling capacity).
- Risk: Inconsistent performance in end applications (e.g., nutraceuticals, cosmetics).
-
Mitigation: Require suppliers to provide detailed specifications (e.g., peptide profile, molecular weight distribution) and conduct batch testing.
-
Source Material Traceability and Purity
- Pitfall: Hydrolysed gelatine derived from bovine, porcine, or piscine sources may carry contamination risks (e.g., BSE/TSE, allergens, heavy metals).
- Risk: Regulatory non-compliance or consumer safety issues.
-
Mitigation: Ensure full traceability of raw materials, certified BSE/TSE-free status, Halal/Kosher certification if required, and adherence to pharmacopeial standards (e.g., Ph. Eur., USP).
-
Residual Enzymes or Processing Aids
- Pitfall: Incomplete removal of proteolytic enzymes used in hydrolysis.
- Risk: Unintended enzymatic activity in final formulations.
-
Mitigation: Request proof of inactivation/removal steps and residual enzyme testing.
-
Microbial Contamination
- Pitfall: Poor hygiene during processing or inadequate drying/storage.
- Risk: High microbial load compromising product safety.
-
Mitigation: Enforce strict microbiological specifications (e.g., total aerobic count, absence of E. coli, Salmonella) and GMP compliance.
-
Lack of Standardized Testing Methods
- Pitfall: Suppliers may use proprietary or non-standardized assays for quality control.
- Risk: Misleading or non-comparable quality data.
- Mitigation: Specify required test methods (e.g., nitrogen content, amino acid profile, solubility) aligned with ISO or AOAC standards.
H2b: Intellectual Property (IP)-Related Pitfalls
- Patented Production Processes or Formulations
- Pitfall: Use of hydrolysed gelatine manufactured via patented enzymatic or thermal processes.
- Risk: Infringement of third-party IP when sourcing or using the material.
-
Mitigation: Conduct freedom-to-operate (FTO) analysis and request IP indemnification clauses in supply agreements.
-
Supplier-Specific Modifications
- Pitfall: “Tailored” hydrolysed gelatine with proprietary modifications (e.g., specific peptide sequences for bioactivity).
- Risk: Dependence on a single supplier and potential IP encumbrance.
-
Mitigation: Clarify ownership of modifications and ensure rights to use, modify, and commercialize the sourced material.
-
Trade Secrets and Lack of Transparency
- Pitfall: Suppliers may withhold critical process details under the guise of trade secrets.
- Risk: Inability to assess quality consistency or regulatory compliance.
-
Mitigation: Negotiate access to redacted process information or third-party audit rights.
-
Brand and Certification Conflicts
- Pitfall: Use of protected terms (e.g., “Bioactive Collagen Peptides®”) without licensing.
- Risk: Trademark infringement and legal action.
-
Mitigation: Verify trademarks and obtain proper licensing if using branded ingredients.
-
Joint Development IP Ownership
- Pitfall: Collaborating with suppliers on custom hydrolysed gelatine development without clear IP agreements.
- Risk: Disputes over ownership of improvements or formulations.
- Mitigation: Define IP ownership upfront in joint development agreements.
Conclusion:
To avoid pitfalls in sourcing hydrolysed gelatine, implement rigorous supplier qualification, demand transparency in quality data and manufacturing processes, and conduct thorough IP due diligence. A robust sourcing strategy should integrate technical specifications, compliance checks, and legal safeguards under the H2 framework (Quality and IP).

Logistics & Compliance Guide for Hydrolysed Gelatine
(HS Code: 3503.00)
H2: Storage and Handling Requirements
Proper storage and handling of hydrolysed gelatine are critical to maintaining product quality, ensuring safety, and complying with international and regional regulations. The following guidelines under H2 address environmental conditions, packaging integrity, and operational best practices.
1. Storage Conditions
-
Temperature:
Store in a cool, dry place at temperatures between 15°C and 25°C (59°F to 77°F). Avoid exposure to excessive heat or freezing conditions, which may degrade protein structure or promote microbial growth. -
Humidity:
Maintain relative humidity below 60%. Hydrolysed gelatine is hygroscopic and can absorb moisture, leading to clumping, microbial contamination, or loss of functionality. -
Ventilation:
Ensure adequate air circulation to prevent moisture buildup and maintain ambient conditions. Avoid storage near steam pipes, boilers, or other heat sources. -
Light Exposure:
Protect from direct sunlight and UV radiation. Prolonged exposure can degrade amino acids and reduce product efficacy. Use opaque or UV-protected packaging when possible. -
Shelf Life:
Typically 24 to 36 months from the date of manufacture when stored under recommended conditions. Always observe the manufacturer’s labeled expiration date.
2. Packaging and Containment
- Use food-grade, moisture-resistant packaging (e.g., multi-layer polyethylene bags with aluminum foil lining) for bulk or retail quantities.
- Ensure packages are hermetically sealed to prevent moisture uptake and contamination.
- For bulk transport, use intermediate bulk containers (IBCs) or flexible intermediate bulk tanks (FIBCs) lined with moisture barriers.
- Label all packaging with:
- Product name: “Hydrolysed Gelatine”
- Batch number
- Manufacturing and expiration dates
- Storage instructions
- Safety and handling symbols (if applicable)
3. Handling Procedures
- Personal Protective Equipment (PPE):
Operators should wear: - Dust masks (to prevent inhalation of fine particles)
- Gloves (nitrile or latex)
- Safety goggles
-
Protective clothing to avoid skin contact
-
Dust Control:
Hydrolysed gelatine powder can generate airborne dust. Use local exhaust ventilation or closed handling systems during transfer and processing to minimize inhalation risk. -
Cross-Contamination Prevention:
- Store separately from non-food-grade chemicals, allergens, or strong-smelling substances.
- Use dedicated equipment and utensils if processing multiple products.
-
Implement strict cleaning protocols between batches.
-
Hygiene Standards:
Adhere to GMP (Good Manufacturing Practices) and HACCP principles. Ensure all handling areas are clean, dry, and pest-free.
4. Regulatory Compliance
-
Food Safety (e.g., FDA, EFSA, FSSAI):
Confirm the hydrolysed gelatine is produced under food-safe conditions and complies with relevant food additive regulations (e.g., EU Regulation (EC) No 1333/2008, FDA 21 CFR §184.1355). -
Allergen Labeling:
Gelatine is derived from animal sources (usually bovine or porcine). Label accordingly to meet allergen declaration requirements in target markets. -
Religious & Dietary Compliance:
- For Halal or Kosher markets, ensure certification from an accredited body.
-
Specify source (bovine, porcine, piscine) on documentation to meet religious and consumer preferences.
-
Import/Export Documentation:
Provide: - Certificate of Analysis (CoA)
- Certificate of Origin
- Halal/Kosher certificates (if applicable)
- Health certificate (required by some countries for animal-derived products)
5. Transportation Considerations
- Use dry, clean, and pest-free cargo compartments.
- Avoid co-loading with hazardous materials, perishables, or moisture-sensitive goods.
- Monitor temperature and humidity during transit, especially in tropical or extreme climates.
- Use pallets with moisture barriers and secure loads to prevent shifting or package damage.
Note: Always refer to the manufacturer’s Safety Data Sheet (SDS) and technical specifications for product-specific guidance. Compliance with local, national, and international standards is mandatory for legal distribution and consumer safety.
Prepared under H2: Storage and Handling Requirements
Conclusion for Sourcing Hydrolyzed Gelatine:
Sourcing high-quality hydrolyzed gelatine requires a comprehensive evaluation of several key factors, including raw material origin (porcine, bovine, or alternative sources), manufacturing processes, regulatory compliance, and supplier reliability. As applications for hydrolyzed gelatine continue to expand across pharmaceuticals, nutraceuticals, cosmetics, and food industries, ensuring product safety, traceability, and consistency becomes paramount. Sustainable and ethical sourcing practices, along with adherence to international quality standards such as Halal, Kosher, and ISO certifications, are increasingly important to meet consumer and regulatory demands. Ultimately, establishing strong partnerships with transparent and audited suppliers will enable organizations to secure a stable supply of effective, safe, and compliant hydrolyzed gelatine that aligns with both quality objectives and market expectations.