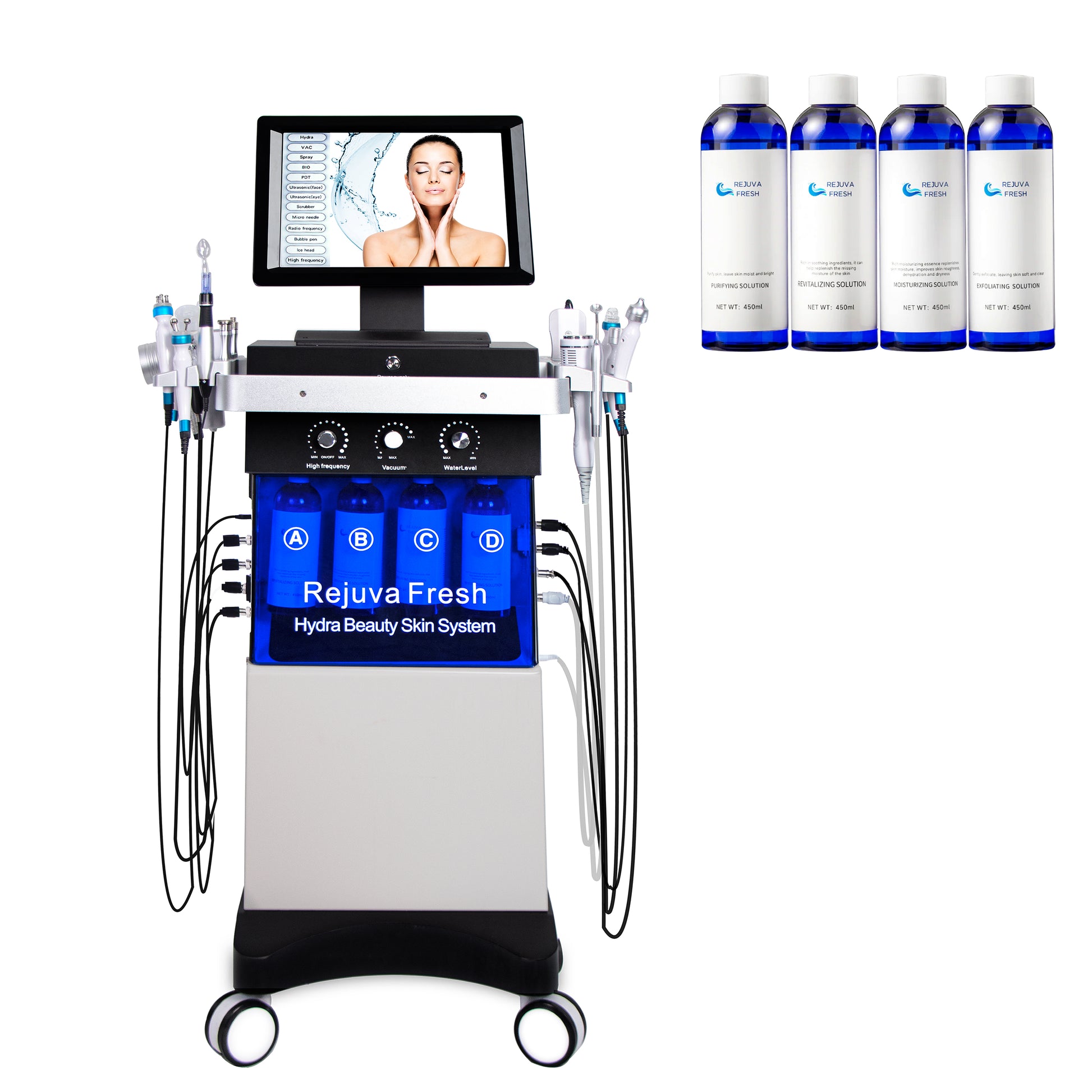
The global skincare devices market is experiencing robust growth, driven by rising consumer demand for non-invasive cosmetic treatments and advancements in aesthetic technology. According to Mordor Intelligence, the facial spa equipment market is projected to grow at a CAGR of over 9.5% from 2023 to 2028, fueled by increasing awareness of skin health and the popularity of procedures like hydrafacials. As one of the most sought-after treatments in dermatology and spa clinics, Hydra Facials combine cleansing, exfoliation, extraction, hydration, and antioxidant protection in a single session — creating significant demand for reliable, high-performance machines. This surge has led to a competitive landscape of manufacturers innovating in precision, automation, and user experience. Based on market presence, technological advancements, FDA compliance, and customer reviews, here are the top 7 Hydra Facial machine manufacturers shaping the future of skin rejuvenation.
Top 7 Hydra Facial Machine Manufacturers 2026
(Ranked by Factory Capability & Trust Score)
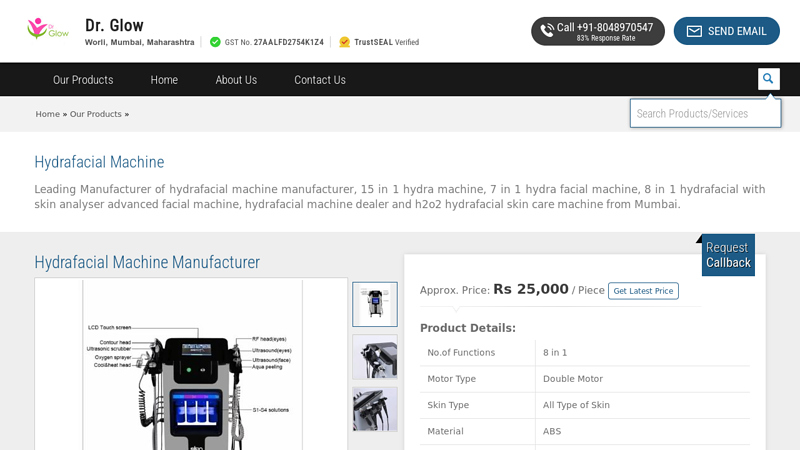
#1 Hydrafacial Machine Manufacturer
Domain Est. 2015
Website: drglowindia.com
Key Highlights: 12 In 1 Hydrafacial Machine · No.of Functions: 10 in 1 · Motor Type: Double Motor · Skin Type: All Type of Skin · Material: ABS · Power: 600 W · Color: White….
#2 DiamondGlow®
Domain Est. 2004
Website: diamondglow.com
Key Highlights: The DiamondGlow® device is a general dermabrasion device that gently removes the top layer of skin and delivers topical cosmetic serums onto the skin. IMPORTANT ……
#3 corporate
Domain Est. 2020
Website: hydrafacialemea.com
Key Highlights: The Hydrafacial system delivers immediate, noticeable results that keep your customers returning regularly. The universally beneficial treatment is safe for ……
#4 Hydra Facial Machine
Domain Est. 2022
Website: beautytherapymachines.com
Key Highlights: 7 In 1 Hydrafacial Machine · No.of Functions: 7 in 1 · Max Plate Size: 4″-12″ · Automation Grade: Automatic · Frequency: 50-60HZ · Output Power: 25W · Vacuum Pressure ……
#5 Hydrafacial™ Elite
Domain Est. 2023
Website: hydrafacialist.lt
Key Highlights: Hydrafacial Elite offers a range of personalised treatments. The brand’s most popular device, recognised by beauty experts worldwide….
#6 Top 100 Hydrafacial Machine Manufacturers in 2025
Domain Est. 2022
Website: ensun.io
Key Highlights: Hydrafacial is a leading manufacturer of skincare technology, renowned for its Vortex Fusion Technology that cleanses, extracts, and hydrates the skin through a ……
#7 Hydrafacial
Domain Est. 2005
Website: hydrafacial.com
Key Highlights: Hydrafacial treatments are the ultimate solution for improving the look of dullness, fine lines and pores, promoting smoother and more radiant-looking skin….
Expert Sourcing Insights for Hydra Facial Machine

H2: 2026 Market Trends for Hydra Facial Machines
The Hydra Facial machine market is poised for significant evolution by 2026, driven by technological advancements, rising consumer demand for non-invasive skincare, and the expansion of aesthetic treatments into mainstream wellness. As the global skincare and beauty industry continues to grow, Hydra Facial machines—known for their hydradermabrasion, chemical peel, and extraction capabilities—are becoming increasingly popular among dermatologists, medspas, and even home users. Several key trends are expected to shape the market landscape in 2026.
1. Technological Innovation and Customization
By 2026, Hydra Facial devices are expected to integrate advanced technologies such as AI-driven skin analysis, IoT-enabled treatment tracking, and personalized serum delivery systems. These features will allow practitioners to offer highly customized treatments based on real-time skin assessments. Brands are likely to incorporate smart sensors and mobile app connectivity to monitor skin progress over time, enhancing client engagement and treatment efficacy.
2. Expansion into At-Home Devices
The demand for professional-grade skincare at home continues to rise. By 2026, the market is anticipated to see a surge in FDA-cleared or dermatologist-approved at-home Hydra Facial devices. These compact, user-friendly machines will offer scaled-down versions of clinical treatments, making hydradermabrasion and infusion therapies more accessible. This trend aligns with the broader shift toward self-care and preventative skincare routines.
3. Growth in Emerging Markets
Asia-Pacific, Latin America, and the Middle East are expected to be high-growth regions for Hydra Facial machines. Increasing disposable incomes, growing awareness of aesthetic treatments, and the influence of social media beauty standards are fueling demand. Local partnerships, multilingual training programs, and region-specific marketing will be critical for global brands aiming to capture market share.
4. Emphasis on Sustainability and Clean Beauty
Consumers in 2026 will increasingly favor eco-conscious brands. As a result, manufacturers of Hydra Facial machines are likely to focus on sustainable materials, refillable serum cartridges, and recyclable packaging. The integration of clean, non-toxic serums compatible with Hydra Facial systems will also become a key differentiator in a competitive market.
5. Integration with Holistic Wellness
The boundary between skincare and wellness is blurring. By 2026, Hydra Facial treatments are expected to be offered in wellness retreats, luxury hotels, and integrative health clinics. Providers may combine Hydra Facial sessions with services like LED therapy, lymphatic drainage, or nutritional counseling, positioning the treatment as part of a comprehensive wellness experience.
6. Increased Competition and Market Consolidation
As the market expands, more players—including both established medical device companies and startups—are expected to enter the space. This will likely lead to increased competition, price pressure, and potential consolidation through mergers and acquisitions. Innovation and brand reputation will be key to maintaining a competitive edge.
In conclusion, the Hydra Facial machine market in 2026 will be shaped by personalization, accessibility, technological integration, and a growing emphasis on sustainability and wellness. Companies that adapt to these trends and invest in R&D, global outreach, and consumer education will be best positioned for long-term success.
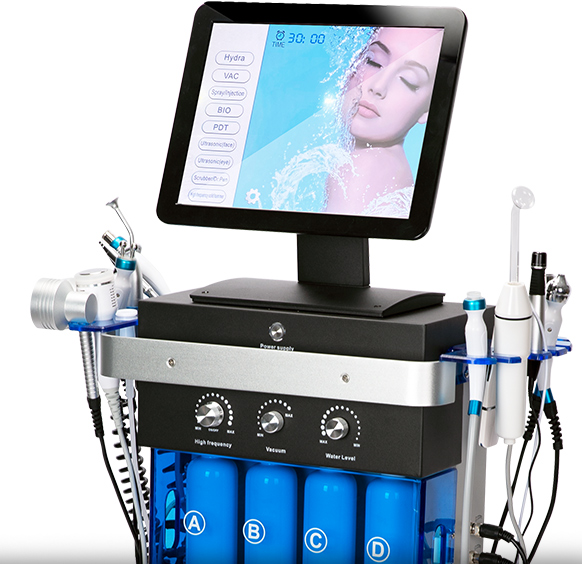
Common Pitfalls When Sourcing a Hydra Facial Machine (Quality and Intellectual Property)
Sourcing a Hydra Facial machine, especially from international or third-party suppliers, comes with several risks related to product quality and intellectual property (IP) infringement. Being aware of these pitfalls can help you avoid costly legal issues, ineffective treatments, and damage to your brand reputation.
Poor Build Quality and Substandard Materials
Many low-cost Hydra Facial machines, particularly those from unverified manufacturers, are built with inferior materials and lack rigorous quality control. This can result in frequent mechanical failures, inconsistent performance, and a shorter device lifespan. Look for machines made with medical-grade components and verify certifications such as CE, ISO 13485, or FDA registration to ensure safety and durability.
Inaccurate or Inconsistent Treatment Performance
A major concern with off-brand Hydra Facial devices is their inability to deliver the same level of exfoliation, extraction, and serum infusion as clinically proven systems. Some machines may lack proper pressure calibration or use lower-grade vacuum motors, leading to ineffective treatments or even skin damage. Always test the machine or request clinical performance data before committing to a purchase.
Lack of Genuine IP Compliance and Risk of Infringement
The term “Hydra Facial” is a registered trademark and the technology is protected by patents held by HydraFacial LLC. Sourcing machines marketed as “Hydra Facial” or “HydraFacial clones” from unauthorized manufacturers can expose you to legal liability for trademark and patent infringement. Even if a device is labeled differently, if it copies patented features (e.g., vortex tip design, spiral suction), it may still violate IP laws.
Misleading Marketing and False Certifications
Some suppliers falsely claim their devices are “FDA-approved” or “equivalent to HydraFacial.” In reality, the FDA does not “approve” most aesthetic devices but rather clears them through a 510(k) process—and only the original HydraFacial devices have undergone this for specific indications. Be cautious of vendors using deceptive language or providing forged certification documents.
Inadequate Technical Support and Spare Parts Availability
Low-cost machines often come with limited or no after-sales support. If the device breaks down, sourcing replacement tips, handpieces, or software updates can be difficult or impossible. This downtime affects your service delivery and client satisfaction. Verify the supplier’s service network and parts availability before purchasing.
Use of Counterfeit or Non-Compatible Treatment Tips
Many third-party machines rely on disposable vortex tips that mimic the patented HydraFacial tip design. These knock-off tips may not only perform poorly but also infringe on intellectual property. Using them can compromise treatment results and expose your business to legal risk, especially in jurisdictions with strong IP enforcement.
Conclusion
To avoid these pitfalls, always source Hydra Facial machines from authorized distributors or manufacturers with verifiable IP rights. Conduct due diligence on certifications, request proof of IP licensing, and prioritize long-term reliability and legal safety over upfront cost savings. Investing in a compliant, high-quality device protects your clients, your practice, and your reputation.

Logistics & Compliance Guide for Hydra Facial Machine
Product Overview and Intended Use
The Hydra Facial Machine is a non-invasive skincare device designed for professional use in dermatology clinics, medical spas, and aesthetic centers. It combines cleansing, exfoliation, extraction, hydration, and antioxidant protection in a single treatment. The device is intended for facial rejuvenation and improving skin texture, tone, and clarity. It is not intended for surgical use or deep tissue treatment.
Regulatory Classification and Certification
The Hydra Facial Machine is classified as a Class II medical device in the United States under the FDA’s 21 CFR 878.4040 (Skin Abrasion Device). It bears the CE marking in accordance with the Medical Devices Regulation (EU) 2017/745, indicating conformity with health, safety, and environmental protection standards for products sold within the European Economic Area. Documentation includes:
– FDA 510(k) clearance number (e.g., KXXXXXX)
– CE Certificate of Conformity
– ISO 13485:2016 Quality Management System certification
Ensure all regional regulatory requirements are met before distribution.
Import and Export Requirements
When shipping Hydra Facial Machines internationally, comply with the following:
– Obtain an Export License if required by the destination country (e.g., certain countries in Asia and the Middle East).
– Provide a Commercial Invoice, Packing List, Bill of Lading/Air Waybill, and Certificate of Origin.
– Include device registration numbers and regulatory clearances with customs documentation.
– Comply with the U.S. Commerce Department’s Export Administration Regulations (EAR) and check if the device requires an ECCN (Export Control Classification Number).
– Confirm import permits or medical device registration with local health authorities (e.g., ANVISA in Brazil, Health Canada, TGA in Australia).
Packaging and Transportation Specifications
- The machine must be shipped in its original protective packaging, including foam inserts and a sturdy outer carton.
- Label packages with “Fragile,” “This Side Up,” and “Protect from Moisture” indicators.
- Ambient shipping temperature should be maintained between 5°C and 40°C (41°F to 104°F).
- Avoid exposure to extreme humidity or direct sunlight.
- Use couriers with experience in handling medical equipment (e.g., DHL Medical Express, FedEx Healthcare Solutions) for time-sensitive deliveries.
Installation and On-Site Compliance
- Installation must be performed by a certified technician or trained biomedical engineer.
- Verify electrical compatibility: 100–240 VAC, 50/60 Hz. Use appropriate power adapters or voltage regulators if needed.
- Ensure the treatment area meets clinical hygiene standards and has access to clean water and proper drainage (if applicable).
- Conduct a post-installation safety and functionality check, and document compliance with local electrical and medical facility codes.
Training and User Certification
- Only licensed skincare professionals or medically supervised personnel may operate the device.
- Mandatory training includes:
- Device operation and safety protocols
- Infection control and sterilization of handpieces
- Emergency shutdown procedures
- Proper disposal of single-use components
- Maintain training records and provide certification to operators. Refresher courses are recommended annually.
Maintenance and Servicing Protocol
- Schedule preventive maintenance every 6 months or after 500 treatment cycles, whichever comes first.
- Use only manufacturer-approved parts and accessories.
- Keep a service log documenting all repairs, calibrations, and software updates.
- Report device malfunctions or adverse events to the manufacturer and relevant regulatory body (e.g., FDA MedWatch, EUDAMED) within required timelines.
Data Privacy and Software Compliance
- The device may collect anonymized treatment data; ensure compliance with data protection laws (e.g., GDPR, HIPAA).
- Do not connect the device to unsecured networks.
- Software updates must be downloaded only from the manufacturer’s secure portal.
- Disable remote access features unless required and secured with strong authentication.
Disposal and End-of-Life Management
- At end-of-life, dispose of the unit in accordance with local electronic waste (WEEE) regulations.
- Remove and separately dispose of batteries or hazardous components per environmental guidelines.
- Data storage components (if any) must be securely erased before disposal.
- Contact the manufacturer for take-back or recycling programs.
Documentation and Recordkeeping
Maintain the following records for a minimum of 10 years:
– Device serial number and purchase date
– Regulatory approvals and certificates
– Installation and maintenance logs
– Operator training records
– Adverse event reports
– Software version history
These records must be available for audit by regulatory authorities upon request.
Conclusion for Sourcing a Hydra Facial Machine:
Sourcing a Hydra Facial machine is a strategic investment that can significantly enhance the service offerings and profitability of a skincare or aesthetic clinic. When making this decision, it is essential to consider factors such as machine quality, regulatory compliance, brand reputation, warranty, after-sales support, and cost-effectiveness. Choosing a reliable supplier or manufacturer—whether domestic or international—ensures access to advanced technology, genuine equipment, and professional training. Additionally, verifying certifications (such as FDA or CE) and assessing customer reviews can mitigate risks associated with counterfeit or substandard devices. Ultimately, a well-researched sourcing decision not only guarantees optimal treatment results and client satisfaction but also supports long-term business growth in the competitive beauty and wellness industry.