The global medical device manufacturing market is witnessing steady expansion, driven by rising demand for minimally invasive surgical instruments—including precision tools such as Fogarty clamps. According to Grand View Research, the global cardiovascular devices market size was valued at USD 54.7 billion in 2022 and is expected to grow at a compound annual growth rate (CAGR) of 6.3% from 2023 to 2030. As a key component in vascular surgery, Fogarty clamps—used primarily for embolectomy procedures—are in increasing demand, prompting growth among specialized manufacturers. Mordor Intelligence projects the medical instruments market will expand at a CAGR of over 6.8% during the forecast period (2023–2028), underscoring the importance of high-quality, reliable instrument production. Within this landscape, a select group of manufacturers has emerged as leaders in engineering, regulatory compliance, and global distribution of Fogarty clamps, setting industry benchmarks for performance and sterility. Below are the top four manufacturers shaping this niche yet critical segment.
Top 4 Fogarty Clamp Manufacturers 2026
(Ranked by Factory Capability & Trust Score)
#1 Atraumatic Occlusion
Domain Est. 1994
Website: edwards.com
Key Highlights: Fogarty® disposable clamp inserts. Clamp insert sets are available in 33, 61 and 86 mm sizes for a wide range of procedures. Not made with natural rubber latex ……
#2 Fogarty safe jaw clamp, 9 1/4” 17
Domain Est. 2003
Website: amblersurgical.com
Key Highlights: Fogarty safe jaw clamp 9 1/4”,curved shanks, angled jaws, ring handle. $815.00. Item# 17-565. Qty. – +. Add to Cart. Fogarty safe jaw clamp, ……
#3 Fogarty Clamps
Domain Est. 2006
Website: artisanmed.com
Key Highlights: Fogarty Clamps ; Fogarty Hemagrip Clamp, angled jaws, 61.0 mm jaw length, 4 5/8″ (11.5 cm). AM55-7525 ; Fogarty Hemagrip Clamp, angled jaws, 61.0 mm jaw length, 8 ……
#4 Fogarty
Domain Est. 2009
Website: novosurgical.com
Key Highlights: Novo Surgical’s Fogarty-Type Hemagrip Clamp is a unique surgical instrument for use in cardiothoracic procedures. This clamp features two wide and flat jaws ……
Expert Sourcing Insights for Fogarty Clamp

H2: 2026 Market Trends for Fogarty Clamp
The market for Fogarty clamps—catheter-based devices primarily used in vascular surgery for embolectomy and thrombectomy procedures—is expected to undergo notable shifts by 2026, driven by advancements in minimally invasive surgery, an aging global population, and rising incidence of cardiovascular diseases. Below is an analysis of key market trends shaping the landscape for Fogarty clamps in 2026:
-
Growing Prevalence of Peripheral Artery Disease (PAD) and Acute Limb Ischemia (ALI)
The increasing global burden of cardiovascular conditions, particularly PAD and ALI, is a primary driver for Fogarty clamp utilization. With aging populations in North America, Europe, and parts of Asia, the demand for endovascular interventions, including balloon catheter embolectomy using Fogarty clamps, is projected to rise steadily through 2026. -
Shift Toward Minimally Invasive Procedures
There is a continued trend favoring minimally invasive surgical techniques over open surgery. While newer technologies like mechanical thrombectomy devices and thrombolytic therapies are gaining traction, Fogarty clamps remain a cost-effective, reliable option—especially in resource-constrained settings and emergency scenarios. Their simplicity and proven efficacy ensure ongoing relevance in hybrid procedural suites. -
Competition from Advanced Endovascular Devices
By 2026, Fogarty clamps face increasing competition from next-generation thrombectomy systems (e.g., rotational, aspiration-based devices) that offer real-time imaging integration and reduced vascular trauma. However, the Fogarty clamp maintains a niche due to its low cost, ease of use, and established track record in both arterial and venous applications. -
Emerging Markets Driving Demand
Expansion in healthcare infrastructure across Latin America, Southeast Asia, and parts of Africa is expected to increase access to vascular interventions. In these regions, the affordability and durability of Fogarty clamps make them a preferred tool, supporting market growth despite technological advances in high-income countries. -
Innovation and Product Enhancements
Manufacturers are responding to clinical feedback with improvements in Fogarty clamp design—such as softer balloon materials, enhanced radiopacity, and multi-size kits—to reduce vessel injury and improve procedural outcomes. These incremental innovations are expected to prolong the device’s clinical utility through 2026 and beyond. -
Regulatory and Reimbursement Landscape
Favorable reimbursement policies for vascular interventions in the U.S. and parts of Europe support procedural volume. However, cost-containment pressures may limit adoption of high-priced alternatives, indirectly benefiting the use of traditional tools like the Fogarty clamp, especially in outpatient and ambulatory surgical centers. -
Training and Skill Retention
As surgical training programs increasingly emphasize endovascular techniques, proficiency in Fogarty embolectomy remains a core skill. The device’s role in teaching vascular surgery fundamentals ensures its continued presence in academic and clinical settings.
Conclusion:
While the Fogarty clamp operates in a competitive and evolving interventional space, its role in 2026 remains secure due to clinical reliability, cost efficiency, and global accessibility. Market growth will be driven by rising vascular disease prevalence and expansion in emerging economies, though adoption may plateau in advanced healthcare systems favoring high-tech alternatives. Strategic innovation and education will be key to maintaining its position in the vascular device ecosystem.
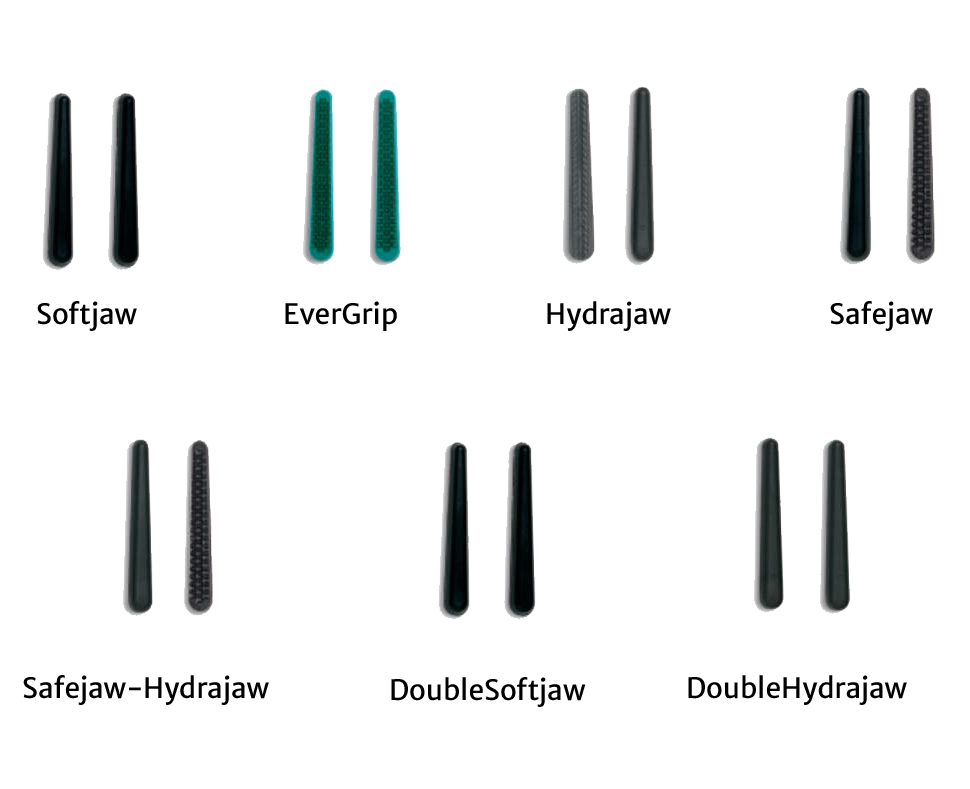
Common Pitfalls When Sourcing Fogarty Clamps (Quality, IP)
Sourcing Fogarty clamps—especially outside of authorized distribution channels—presents significant risks related to both product quality and intellectual property (IP) rights. Being aware of these pitfalls is crucial for healthcare providers, distributors, and procurement teams.
Quality Risks from Unverified Suppliers
One of the most pressing concerns when sourcing Fogarty clamps is the risk of receiving substandard or counterfeit devices. Fogarty clamps are precision medical instruments used in vascular procedures, and compromised quality can lead to serious patient safety issues.
- Counterfeit or Non-Compliant Devices: Unofficial suppliers may offer products that mimic the appearance of genuine Fogarty clamps but are manufactured using inferior materials or processes. These devices may not meet ISO 13485 or FDA standards, increasing the risk of mechanical failure during surgery.
- Lack of Sterility and Packaging Integrity: Improperly sterilized or re-packaged clamps can pose infection risks. Some third-party vendors may repackage single-use devices, violating safety protocols.
- Inconsistent Performance: Poor manufacturing tolerances in non-genuine clamps can affect the balloon inflation control and catheter flexibility, directly impacting surgical outcomes.
Intellectual Property (IP) Infringement
The Fogarty clamp is a trademarked and patented medical device, originally developed by Dr. Thomas J. Fogarty and commercialized by companies like Edwards Lifesciences. Sourcing from unauthorized manufacturers often involves IP violations.
- Trademark and Patent Infringement: Many knockoff versions use names or designs that closely resemble the original Fogarty brand, infringing on registered trademarks and design patents. Purchasing such products—even unknowingly—can expose organizations to legal liability.
- Gray Market and Unauthorized Distribution: Devices sourced through gray market channels may be diverted from legitimate supply chains, potentially violating distribution agreements and IP protections. These products lack traceability and post-market support.
- Reputational and Regulatory Risk: Institutions found using or distributing IP-infringing medical devices may face regulatory scrutiny, damage to reputation, and loss of accreditation.
Mitigation Strategies
To avoid these pitfalls, always procure Fogarty clamps through authorized distributors or directly from the IP holder. Verify supplier credentials, request documentation (e.g., Certificates of Conformity, traceability records), and conduct audits when sourcing in bulk. Prioritizing compliance and patient safety over cost savings is essential in medical device procurement.

Logistics & Compliance Guide for Fogarty Clamp
This guide outlines essential logistics and compliance considerations for the handling, transportation, storage, and regulatory requirements associated with the Fogarty Clamp, a medical device used in vascular procedures for embolectomy or thrombectomy.
Regulatory Classification and Approvals
The Fogarty Clamp is classified as a Class II medical device under the U.S. Food and Drug Administration (FDA) regulations, typically falling under product code FAB or NEV. It requires 510(k) premarket notification clearance. Equivalent regulatory approvals must be secured in other markets, such as CE marking in the European Union under the Medical Device Regulation (MDR) 2017/745, or compliance with Health Canada’s Medical Devices Regulations. Always verify the specific clearance status and labeling requirements for the intended market before distribution.
Labeling and Packaging Standards
All packaging must include clear labeling compliant with ISO 15223-1 and applicable regional standards. Labels must display the device name (Fogarty Clamp), model and size, lot number, expiration date (if applicable), sterile barrier integrity indicators, and single-use designation. Packaging must maintain sterility throughout distribution and comply with ISO 11607 for materials and sealing integrity. Include UDI (Unique Device Identification) in both human-readable and machine-readable (e.g., barcode or Data Matrix) formats per FDA and EU MDR requirements.
Sterility and Single-Use Compliance
The Fogarty Clamp is intended for single-use only and must be supplied sterile. Any breach in the sterile barrier constitutes a critical failure, and the device must not be used. Reuse, reprocessing, or resterilization is strictly prohibited and violates FDA, EU, and manufacturer guidelines. Distributors and healthcare providers must implement protocols to prevent misuse and ensure traceability of single-use devices.
Storage and Environmental Conditions
Store the Fogarty Clamp in a clean, dry environment with temperatures between 15°C and 30°C (59°F to 86°F) and relative humidity below 60%. Avoid exposure to direct sunlight, radiation, or extreme temperatures. Devices must remain in their original sealed packaging until point of use to ensure sterility and performance integrity.
Transportation and Handling
Transport the device using validated shipping methods that protect against physical damage, moisture, and temperature extremes. Use temperature-controlled logistics if required by regional regulations or environmental risks. Packages must withstand standard distribution hazards as defined in ISTA 3A or equivalent standards. Maintain chain-of-custody documentation, especially for high-value or temperature-sensitive shipments.
Import and Export Documentation
For international distribution, ensure all shipments include a Certificate of Free Sale (CFS), Certificate of Conformance (CoC), and, where required, an Export Certificate per FDA 21 CFR Part 807. Exported devices must comply with the importing country’s regulatory framework. Accurate Harmonized System (HS) codes (e.g., 9021.21 for surgical instruments) must be used for customs clearance.
Post-Market Surveillance and Adverse Event Reporting
Manufacturers and authorized representatives are responsible for monitoring post-market performance. Any adverse events, device malfunctions, or suspected counterfeit products involving the Fogarty Clamp must be reported promptly to relevant regulatory bodies—e.g., FDA MedWatch (Form 3500A), EUDAMED in the EU, or Canada’s Medical Device Incident Reporting system. Maintain records for a minimum of 10 years post-device withdrawal.
Training and Distribution Controls
Ensure all logistics personnel, distributors, and healthcare users receive training on proper handling, storage, and compliance with single-use and sterility protocols. Implement quality agreements with distributors to enforce compliance with regulatory and logistical standards. Unauthorized third-party distribution must be prevented to maintain product integrity and regulatory compliance.
Environmental and Disposal Compliance
After use, the Fogarty Clamp must be treated as medical waste and disposed of in accordance with local biohazard and medical waste regulations (e.g., OSHA, EPA, or EU Directive 2008/98/EC). Do not dispose of in general waste streams. Provide disposal guidance in product packaging or instructions for use (IFU).
Conclusion for Sourcing Fogarty Clamp:
After a thorough evaluation of suppliers, product specifications, regulatory compliance, cost, and availability, sourcing the Fogarty clamp requires careful consideration to ensure quality, reliability, and patient safety. The Fogarty clamp, commonly used in vascular and surgical procedures, is a critical medical device where performance and sterility are paramount. It is recommended to partner with certified manufacturers or authorized distributors that comply with ISO standards and FDA regulations. Prioritizing suppliers with a proven track record, consistent quality control, and dependable delivery timelines will mitigate risks associated with device failure or supply chain disruptions. Additionally, conducting regular audits and maintaining strong supplier relationships will support long-term success. Ultimately, the decision should balance cost-efficiency with uncompromised clinical safety and adherence to medical device standards.