The global dental care market is experiencing robust growth, driven by rising awareness of oral hygiene and increasing demand for preventive dental products. According to Grand View Research, the global dental devices market size was valued at USD 39.5 billion in 2023 and is expected to expand at a compound annual growth rate (CAGR) of 7.2% from 2024 to 2030. This growth trajectory is mirrored in niche segments such as interdental cleaning tools, where innovation and accessibility are reshaping consumer preferences. Floss bridge threaders, in particular, have emerged as essential aids for individuals with braces, bridges, or tight dental work, fueling demand among both consumers and dental professionals. As the market expands, a select group of manufacturers has distinguished itself through product quality, ergonomic design, and clinical validation. Here, we examine the top eight floss bridge threader manufacturers leading this segment, evaluating their market presence, distribution networks, and performance based on industry data and consumer insights.
Top 8 Floss Bridge Threader Manufacturers 2026
(Ranked by Factory Capability & Trust Score)
#1 BridgeAid® Floss Threader
Domain Est. 1995
Website: henryschein.com
Key Highlights: BridgeAid® Floss Threader. Used for flossing under bridges and braces, the Unique BRIDGEAID Dental Floss Threader is endorsed by oral hygienists worldwide….
#2 Oral
Domain Est. 1995
Website: oralb.com
Key Highlights: Rating 1.6 (321) · Free delivery · 60-day returns…
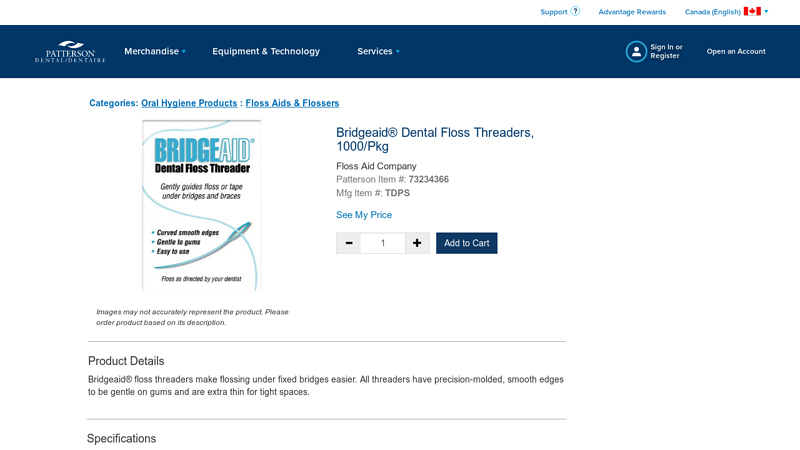
#3 Bridgeaid® Dental Floss Threaders, 1000/Pkg
Domain Est. 1996
Website: pattersondental.com
Key Highlights: Bridgeaid floss threaders make flossing under fixed bridges easier. All threaders have precision-molded, smooth edges to be gentle on gums and are extra thin ……
#4 Floss threaders
Domain Est. 1999
Website: net32.com
Key Highlights: 30-day returnsFloss threaders. We offer you great deals off retail pricing on top selling dental brands. Navigate below to find discounts on all your Floss threaders needs….
#5 Floss Threaders
Domain Est. 2002
Website: tristatedental.com
Key Highlights: 30-day returnsFloss Threaders ; Bridge Aid Floss Threaders (1000) · 131-TDPS ; G-U-M Flossmate Floss Handle (12) · 130-845P ; G-U-M Ez Thru Flossers (48) · 130-891P ; G-U-M Crayola…
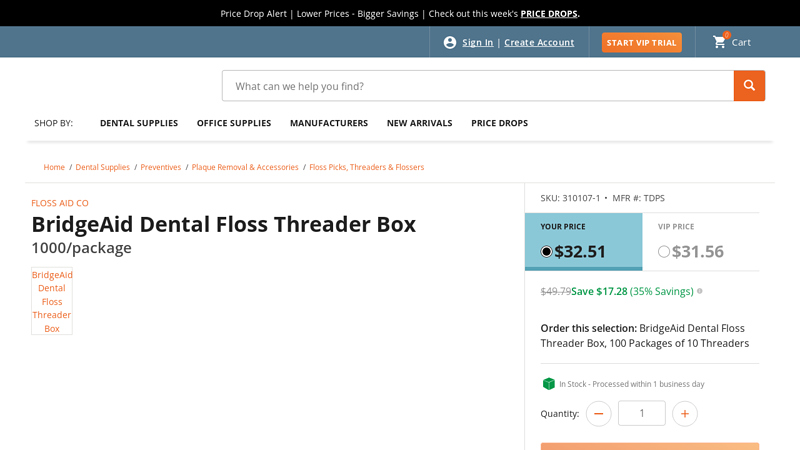
#6 BridgeAid Dental Floss Threader Box
Domain Est. 2003
Website: tdsc.com
Key Highlights: In stock Rating 4.5 33 BridgeAid Dental Floss Threader Box, 100 Packages of 10 Threaders….
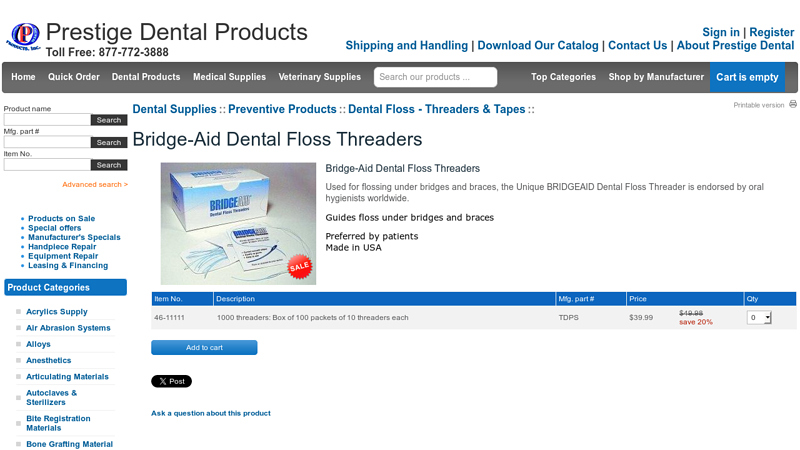
#7 Bridge
Domain Est. 2006
Website: prestigedentalproducts.com
Key Highlights: In stock Free deliveryUsed for flossing under bridges and braces, the Unique BRIDGEAID Dental Floss Threader is endorsed by oral hygienists worldwide….
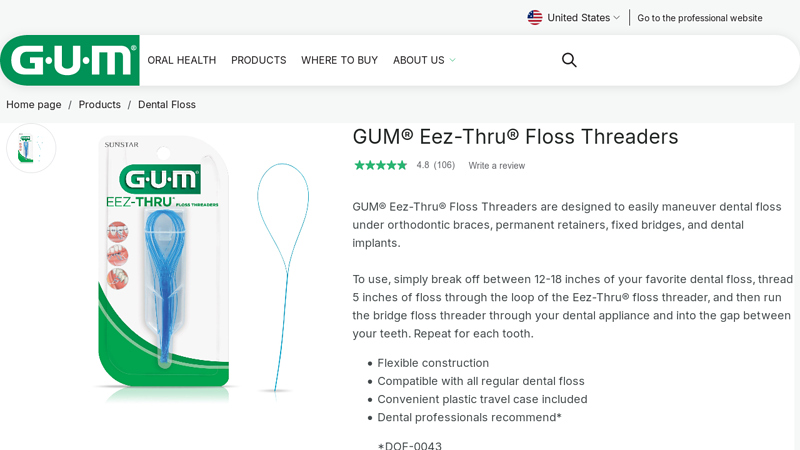
#8 GUM® Eez
Domain Est. 2007
Expert Sourcing Insights for Floss Bridge Threader

H2: 2026 Market Trends for Floss Bridge Threader
The global market for oral care products is projected to experience steady growth through 2026, with increasing consumer emphasis on preventive dental hygiene and the rising prevalence of gum disease and orthodontic treatments. Within this landscape, the Floss Bridge Threader—a specialized dental tool designed to assist individuals with braces, bridges, implants, or tight dental spacing—emerges as a niche yet increasingly significant product category.
-
Rising Demand Driven by Orthodontic Growth
The global orthodontic devices market is expanding, fueled by growing aesthetic consciousness and increased access to orthodontic care, especially among adults. As of 2026, a larger segment of the population will be wearing braces or retainers, increasing the need for effective interdental cleaning tools. Floss bridge threaders are essential for navigating complex dental work, positioning them as a must-have accessory in daily oral hygiene routines. -
Innovation and Product Differentiation
Manufacturers are expected to focus on ergonomic design, biodegradable materials, and multi-functional features (e.g., integrated picks or reusable handles). By 2026, smart packaging with instructional guides and subscription models could enhance user adherence and brand loyalty. Additionally, integration with oral care apps that track flossing habits may provide a competitive edge. -
Expansion in E-Commerce and Direct-to-Consumer Channels
With the continued rise of online health and wellness retail, floss bridge threaders will benefit from targeted digital marketing, subscription services, and bundling with electric flossers or water flossers. Platforms like Amazon, Walmart.com, and dental e-commerce sites will play a key role in market penetration, especially during post-pandemic shifts toward home-based dental care. -
Growing Awareness and Preventive Care Initiatives
Public health campaigns and dental professional endorsements will continue to promote interdental cleaning as critical for preventing periodontal disease. Dentists and hygienists are likely to recommend floss threaders more frequently, especially for patients with fixed prosthetics. This professional validation will drive consumer trust and adoption. -
Sustainability and Eco-Friendly Materials
By 2026, environmental concerns will influence product development. Biodegradable or compostable floss threaders made from plant-based plastics or reusable stainless steel models may gain traction, aligning with consumer demand for sustainable oral care solutions. -
Regional Market Dynamics
North America and Europe will remain dominant markets due to high dental care spending and awareness. However, the Asia-Pacific region is expected to witness the fastest growth, driven by urbanization, rising disposable incomes, and expanding dental infrastructure in countries like India and China.
In conclusion, the 2026 market for floss bridge threaders will be shaped by technological innovation, preventive healthcare trends, e-commerce expansion, and sustainability. As personalized oral care gains momentum, floss bridge threaders are poised to transition from a niche tool to a mainstream component of comprehensive dental hygiene regimens.
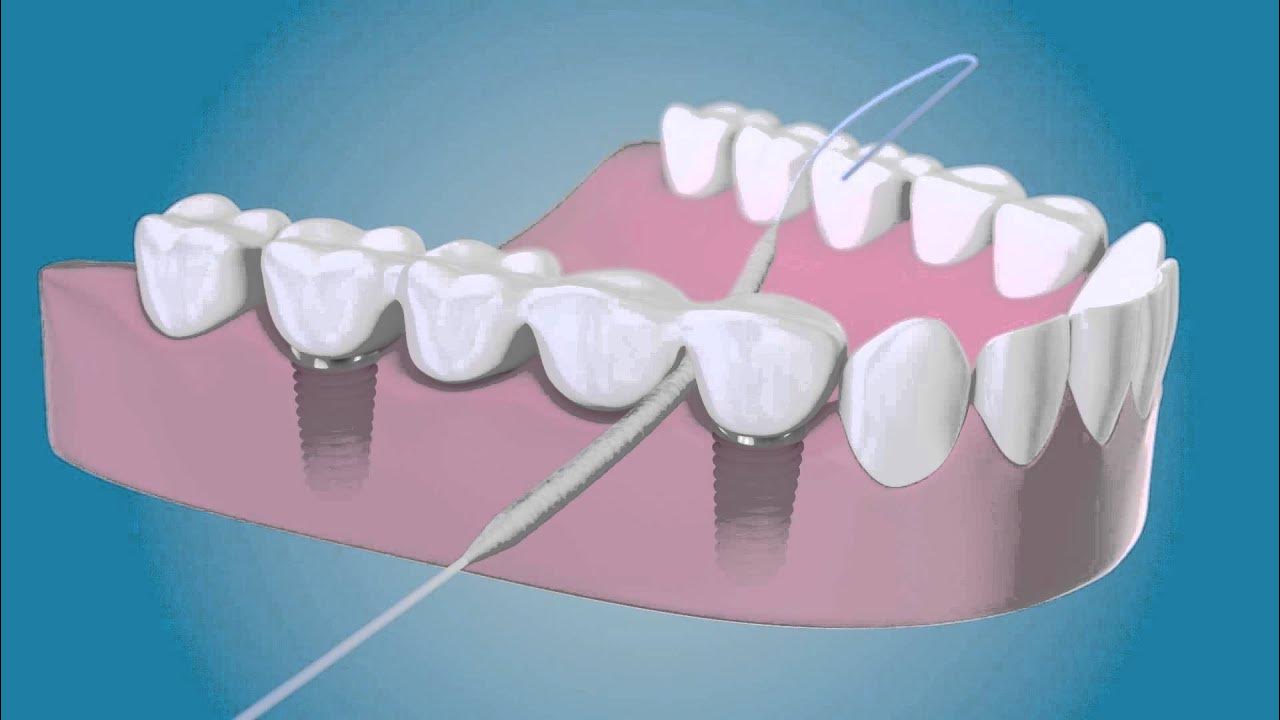
Common Pitfalls When Sourcing Floss Bridge Threaders (Quality and Intellectual Property)
Sourcing Floss Bridge Threaders—especially from overseas manufacturers—can present significant challenges related to product quality and intellectual property (IP) protection. Being aware of these pitfalls is crucial for maintaining customer trust, avoiding legal issues, and ensuring long-term brand success.
Inconsistent or Substandard Material Quality
One of the most frequent issues is receiving threaders made from inferior materials. Low-grade plastics may be brittle, prone to snapping during use, or have rough edges that can damage dental work or irritate gums. Inconsistent wall thickness or poor molding can compromise the structural integrity of the device, leading to product failure and potential safety concerns.
Poor Manufacturing Tolerances and Design Flaws
Even if the raw material is adequate, imprecise manufacturing can result in threaders that don’t function as intended. Misaligned guides, inconsistent hole sizing, or warped shapes can make it difficult or impossible to thread dental floss effectively. These flaws often stem from low-cost molds or inadequate quality control on the production line.
Lack of Sterility and Hygiene Compliance
Dental care products must meet strict hygiene standards. Sourcing from suppliers without proper certifications (such as ISO 13485 for medical devices) may result in non-sterile packaging or manufacturing environments that introduce contaminants. This risk is especially critical if the product is marketed for clinical or professional use.
Infringement of Existing Patents or Designs
Many Floss Bridge Threader designs are protected by utility or design patents. Sourcing a product that closely mimics a patented design—even unintentionally—can lead to legal disputes, cease-and-desist orders, or costly litigation. Conducting thorough IP due diligence before finalizing a supplier is essential to avoid infringing on existing intellectual property rights.
Copycat Products and Trademark Violations
Some suppliers may offer threaders that copy branded designs or include logos and trademarks without authorization. Using such products exposes your business to trademark infringement claims. Always verify that the design is either generic, licensed, or original to avoid legal liability.
Inadequate Supplier Verification and Transparency
Suppliers may claim compliance with quality standards or IP legitimacy without providing verifiable documentation. Relying on marketing materials alone—without audits, samples, or third-party testing—increases the risk of receiving non-compliant or counterfeit products. Always request certifications, conduct factory audits, and perform independent quality checks.
Limited Accountability and Recourse
When sourcing internationally, enforcing quality standards or intellectual property rights can be difficult and expensive. Legal jurisdiction, language barriers, and lack of enforceable contracts may leave buyers with little recourse if a supplier delivers defective products or violates IP agreements.
Conclusion
To mitigate these risks, conduct thorough due diligence on suppliers, request prototypes and certifications, perform IP clearance searches, and consider working with legal and technical experts familiar with medical or dental device regulations. Prioritizing quality and IP integrity from the outset protects both your brand and your customers.

Logistics & Compliance Guide for Floss Bridge Threader
Product Classification and Regulatory Status
The Floss Bridge Threader is classified as a dental hygiene device intended to assist users in threading dental floss under dental bridges, implants, or other fixed prosthetics. It is typically categorized as a Class I medical device under the U.S. Food and Drug Administration (FDA) regulations, exempt from premarket notification (510(k)) provided it meets general controls and is not intended for surgical use. Ensure compliance with 21 CFR 872.6050 (Dental Floss) and applicable labeling requirements.
Manufacturing and Quality Standards
Manufacturers must adhere to Quality Management System (QMS) requirements such as ISO 13485:2016 for medical devices. Production facilities should implement Good Manufacturing Practices (GMP) to ensure product consistency, sterility (if applicable), and safety. Routine quality audits, process validation, and documentation of corrective actions are required to maintain compliance.
Labeling and Packaging Requirements
All product labeling must comply with FDA 21 CFR Part 801 and relevant international standards (e.g., EU MDR, Health Canada). Labels must include:
– Product name: “Floss Bridge Threader”
– Intended use statement
– Manufacturer or distributor name and address
– Lot or batch number
– Expiration date (if applicable)
– Single-use or reusable designation
– Sterility status (if sterile)
– Language requirements for target markets (e.g., bilingual labeling in Canada)
Primary packaging must protect the device from contamination and damage during shipping and storage. Use tamper-evident seals where appropriate.
Import/Export Regulations
When exporting, verify compliance with destination country regulations:
– United States: FDA registration and listing; no 510(k) required if within exemption scope
– European Union: CE marking under Medical Device Regulation (EU) 2017/745; involve a Notified Body if required
– Canada: Medical Device License (MDL) from Health Canada; classification as Class I
– Australia: Entry in the Australian Register of Therapeutic Goods (ARTG)
Ensure Harmonized System (HS) code 9023.00 (instruments and appliances used in dentistry) is correctly applied for customs clearance.
Shipping and Distribution Logistics
- Use validated packaging to withstand temperature, humidity, and physical stress during transit
- Maintain distribution records for traceability (UDI compliance where applicable)
- Partner with carriers experienced in medical device logistics
- Monitor cold chain requirements if the product is sterility-packaged or sensitive to environmental conditions
Post-Market Surveillance and Reporting
Implement a post-market surveillance system to collect and review customer complaints, adverse events, and product malfunctions. Report device-related deaths, serious injuries, or malfunctions to the FDA via MedWatch (Form 3500A) within required timeframes. Maintain a U.S. Agent if the manufacturer is based outside the United States.
Environmental and Sustainability Compliance
Ensure packaging materials comply with environmental regulations such as REACH (EU), RoHS (if electronic components are present), and local waste disposal laws. Minimize single-use plastics where possible and consider recyclable or biodegradable materials to meet growing sustainability expectations.
Training and Documentation
Provide clear Instructions for Use (IFU) in all supported languages. Train customer service and distribution staff on compliance protocols, recall procedures, and proper handling. Maintain technical files, design history, and regulatory submissions for minimum of 5 years post-product discontinuation (longer in EU under MDR).
Conclusion for Sourcing Floss Bridge Threader:
After a thorough evaluation of potential suppliers, product quality, cost-efficiency, and regulatory compliance, sourcing floss bridge threaders from a reliable and certified manufacturer ensures consistency, safety, and customer satisfaction. Prioritizing suppliers with a proven track record in dental hygiene product manufacturing, adherence to ISO and FDA standards, and strong logistical support will optimize supply chain efficiency. Additionally, considering customization options, sustainable materials, and bulk pricing can enhance brand value and reduce long-term costs. Ultimately, establishing a strategic partnership with a qualified supplier will support product reliability, scalability, and market competitiveness in the oral care industry.