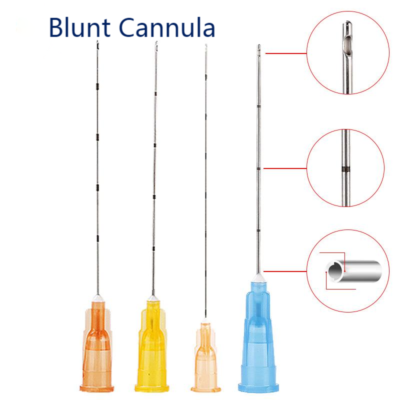
The global demand for blunt-tip needles has surged in recent years, driven by increasing emphasis on safety and precision in cosmetic, dermatological, and medical procedures. According to a 2023 report by Mordor Intelligence, the global hypodermic needles market—which includes blunt-tip variants—is projected to grow at a CAGR of 7.8% from 2023 to 2028. This growth is fueled by rising adoption in aesthetic medicine, where blunt-tip cannulas reduce the risk of bruising, vascular injury, and tissue trauma compared to traditional sharp needles. Additionally, Grand View Research estimates that the global aesthetic devices market reached USD 8.9 billion in 2022 and is expected to expand at a CAGR of 10.3% through 2030, further amplifying the need for high-quality, reliable blunt-tip solutions. As clinics and practitioners prioritize patient safety and procedural efficiency, sourcing from reputable manufacturers has become critical. Based on product quality, regulatory compliance, innovation, and market presence, the following eight manufacturers stand out as industry leaders in the production of blunt-tip needles and cannulas.
Top 8 Colling Blunt Tip Manufacturers 2026
(Ranked by Factory Capability & Trust Score)
#1 HOT ! 2025 Factory Disposable Blunt
Domain Est. 2022
Website: fiathletisme.com
Key Highlights: Rating 4.5 (14) HOT ! 2025 Factory Disposable Blunt-tip Cannula 22g 50mm 70mm Blunt Fine Micro Body Piercing Needles Cannula Syringe Tool · Pick up at store (free shipping).Missi…
#2 McMaster
Domain Est. 1994
Website: mcmaster.com
Key Highlights: McMaster-Carr is the complete source for your plant with over 700000 products. 98% of products ordered ship from stock and deliver same or next day….
#3 Blunt Fill Cannulas & Needles
Domain Est. 1996
Website: cardinalhealth.com
Key Highlights: Cardinal Health offers a line of Monoject™ Blunt Cannulas and Syringes with Plastic Hub, Monoject™ Blunt Cannulas with Metal Hub, Monoject™ Blunt Fill Needles, ……
#4 Laboratory Syringes
Domain Est. 1998
Website: hamiltoncompany.com
Key Highlights: A Hamilton syringe delivers the highest possible performance. Compared to other laboratory syringes, our glass syringes are built to last….
#5 PerkinElmer
Domain Est. 1998
Website: perkinelmer.com
Key Highlights: We believe in the power of science to transform our world. · We drive efficiency and empower sustainable scientific discovery with nearly 90 years of pioneering ……
#6 [PDF] Claimant Handbook
Domain Est. 2001
Website: dol.georgia.gov
Key Highlights: INTERPRETER SERVICES. The Georgia Department of Labor (GDOL) will provide an interpreter for the hearing or voice impaired and for those individuals with ……
#7 NRS: CHAPTER 613
Domain Est. 2003
Website: leg.state.nv.us
Key Highlights: NRS 613.150 Transportation company compelling purchase of uniform from particular person or employer as condition of continuing employment unlawful; penalty….
#8 Individual Income Tax Information
Domain Est. 2004
Website: azdor.gov
Key Highlights: Taxpayers can call (602) 255-3381, and, after making the language selection, select Option 2 for refund status. Taxpayers should have their tax information ……
Expert Sourcing Insights for Colling Blunt Tip

H2: 2026 Market Trends for Colling Blunt Tip
The global market for Colling Blunt Tip devices—commonly used in minimally invasive medical procedures such as electrosurgery and aesthetic treatments—is expected to experience significant evolution by 2026, driven by advancements in medical technology, increasing demand for precision-based surgical tools, and a growing focus on patient safety and recovery times.
-
Rising Adoption in Aesthetic and Dermatological Procedures
By 2026, the aesthetic and dermatology sectors are projected to be major growth drivers for Colling Blunt Tip instruments. These devices are increasingly favored for their ability to deliver controlled thermal energy without damaging surrounding tissues. With the global rise in non-invasive cosmetic procedures—including skin tightening and scar revision—demand for precision tools like the Colling Blunt Tip is expected to surge, particularly in North America, Europe, and parts of Asia-Pacific. -
Technological Innovation and Integration
Manufacturers are anticipated to enhance the functionality of Colling Blunt Tip devices through integration with smart technologies, such as real-time temperature monitoring and feedback systems. These advancements will improve procedural accuracy and reduce complications, aligning with broader healthcare trends toward digitalization and data-driven surgery. By 2026, next-generation blunt tip systems may feature IoT connectivity and AI-assisted guidance, further differentiating them in competitive markets. -
Regulatory and Safety Standards
As regulatory bodies like the FDA and EMA continue to emphasize device safety, Colling Blunt Tip products that demonstrate superior safety profiles—such as reduced risk of tissue perforation or post-operative scarring—are likely to gain market preference. Compliance with updated medical device regulations (e.g., EU MDR) will be critical for market access, potentially consolidating the industry around a smaller number of high-quality manufacturers. -
Expansion in Emerging Markets
Developing regions, including Latin America, Southeast Asia, and the Middle East, are expected to witness increased adoption of advanced electrosurgical tools by 2026. Rising healthcare expenditure, improved medical infrastructure, and growing awareness of minimally invasive techniques will create new opportunities for Colling Blunt Tip manufacturers. Strategic partnerships with local distributors and training initiatives for medical professionals will be key to market penetration. -
Sustainability and Single-Use vs. Reusable Debate
Environmental concerns and infection control protocols are fueling debate over single-use versus reusable medical devices. While reusable Colling Blunt Tips offer cost savings, single-use variants minimize cross-contamination risks. By 2026, market trends may lean toward hybrid models—sterilizable, durable designs with replaceable tips—balancing cost-efficiency, safety, and sustainability.
In summary, the 2026 market landscape for Colling Blunt Tip devices will be characterized by innovation, expanded clinical applications, and geographic diversification. Stakeholders who prioritize safety, technological integration, and regulatory compliance are poised to lead in this evolving sector.

Common Pitfalls When Sourcing Colling Blunt Tip (Quality, IP)
Sourcing medical or laboratory components such as the Colling Blunt Tip requires careful attention to both quality and intellectual property (IP) considerations. Overlooking these aspects can lead to significant risks, including product failure, regulatory non-compliance, and legal liabilities. Below are common pitfalls to avoid:
Poor Quality Control and Inconsistent Manufacturing
One of the most frequent issues when sourcing Colling Blunt Tips—especially from third-party or offshore manufacturers—is variability in product quality. Inferior materials, imprecise tip geometry, or inconsistent wall thickness can compromise performance, leading to leaks, tissue damage, or inaccurate fluid delivery in clinical or research settings. Always verify that suppliers adhere to ISO 13485 or equivalent standards and request batch-specific quality documentation.
Lack of Regulatory Compliance
Colling Blunt Tips used in medical applications may fall under regulatory oversight (e.g., FDA, CE marking). Sourcing from manufacturers that do not comply with applicable medical device regulations can expose your organization to legal and market access risks. Ensure the supplier can provide technical files, conformity declarations, and proper labeling aligned with your target market’s requirements.
Intellectual Property Infringement
The design of the Colling Blunt Tip may be protected by patents or trade secrets. Sourcing generic or “compatible” versions from unauthorized manufacturers risks infringing on existing IP rights. This could result in cease-and-desist orders, product seizures, or costly litigation. Conduct thorough IP due diligence: verify patent status in relevant jurisdictions and confirm the supplier has the right to manufacture and sell the product.
Inadequate Traceability and Documentation
Reliable sourcing requires full traceability—from raw materials to finished goods. Suppliers that lack proper documentation (e.g., material certifications, lot numbers, sterilization records) make it difficult to respond to audits or investigate field failures. Insist on suppliers who maintain a robust quality management system (QMS) with complete documentation trails.
Hidden Costs from Rework or Recalls
Choosing a lower-cost supplier without vetting their quality systems can lead to hidden expenses. Substandard tips may require inspection, rework, or even full product recalls. These downstream costs often outweigh initial savings. Perform cost-of-quality analyses and consider total lifecycle costs, not just unit price.
Supply Chain Vulnerability
Relying on a single or geographically concentrated supplier increases risk of disruption. Evaluate supplier stability, production capacity, and business continuity plans to ensure consistent availability, especially for mission-critical applications.
By proactively addressing these pitfalls—through rigorous supplier qualification, IP validation, and ongoing quality monitoring—organizations can mitigate risks and ensure reliable, compliant sourcing of Colling Blunt Tips.

Logistics & Compliance Guide for Colling Blunt Tip
Product Overview
The Colling Blunt Tip is a medical device designed for safe and precise tissue dissection in surgical procedures. It features a non-penetrating tip to minimize the risk of unintended punctures or damage to surrounding structures. This guide outlines key logistics and regulatory compliance considerations for the distribution, handling, and use of this device.
Regulatory Classification
The Colling Blunt Tip is classified as a Class I medical device under the U.S. Food and Drug Administration (FDA) regulations (21 CFR 878.4800 – Surgical Instruments). It is non-sterile, reusable, and intended for general surgical use. It is exempt from premarket notification (510(k)) but must comply with general controls, including registration, labeling, and quality system requirements.
International Compliance
- European Union (EU): CE marked in accordance with Regulation (EU) 2017/745 (MDR). Classified as Class I (non-sterile, non-measuring).
- Canada: Licensed under Health Canada’s Medical Devices Regulations (SOR/98-282), Class I device.
- Australia: Listed on the Australian Register of Therapeutic Goods (ARTG) as a Class I device.
- Other Markets: Confirm local regulatory requirements prior to import, including registration with national health authorities and adherence to labeling standards.
Labeling Requirements
All packaging and product labeling must include:
– Device name: “Colling Blunt Tip”
– Manufacturer name and address
– Lot number and serial number (if applicable)
– “Rx Only” or “For Surgical Use Only” statement
– Reusable device symbol (if applicable)
– Instructions for use (IFU) in the local language for international shipments
– UDI (Unique Device Identifier) compliance per FDA and EU MDR requirements
Packaging and Shipping
- Packaged in tamper-evident, durable polyethylene bags suitable for repeated sterilization.
- Bulk packaging for institutional orders: 10 or 25 units per sealed inner pouch, multiple pouches per corrugated master carton.
- Ship at ambient temperature; no special handling required.
- Ensure packaging meets ISTA 3A standards for transport durability.
- Include packing slip, COA (Certificate of Conformance), and IFU with each shipment.
Storage Conditions
- Store in a clean, dry environment.
- Temperature range: 15°C to 30°C (59°F to 86°F)
- Humidity: 30% to 60% non-condensing
- Protect from direct sunlight and chemical exposure
Sterilization and Reuse
- Device is sold non-sterile and must be sterilized prior to first use.
- Compatible sterilization methods:
- Steam autoclaving (121°C for 15–20 minutes or 134°C for 3–4 minutes)
- Ethylene oxide (EtO)
- Follow IFU for cleaning, decontamination, and inspection prior to reuse.
- Implement tracking of reprocessing cycles as per facility protocol.
Import and Customs Documentation
For international shipments, include:
– Commercial invoice with HS code (typically 9018.90 – Other surgical instruments)
– Certificate of Manufacture and Conformance
– FDA Export Certificate (for non-U.S. destinations)
– CE Certificate of Conformity (for EU-bound shipments)
– UDI-DI (Device Identifier) on all shipping labels and documentation as required
Quality System Compliance
Manufacturer adheres to:
– ISO 13485:2016 Quality Management System
– FDA 21 CFR Part 820 (Quality System Regulation)
– EU MDR Annex IX, Chapter I (for Class I reusable devices)
– Regular internal audits and management reviews conducted
Adverse Event Reporting
- Report any device-related adverse events or malfunctions to the manufacturer within 10 calendar days.
- In the U.S., manufacturers are required to report to FDA via the MedWatch program (Form 3500A).
- In the EU, incidents must be reported through the Eudamed system per MDR Article 87.
Contact Information
For compliance, logistics, or technical support inquiries:
Colling Medical Devices Inc.
Regulatory Affairs Department
Email: [email protected]
Phone: +1 (800) 555-1234
Address: 123 MedTech Drive, Suite 200, Boston, MA 02110, USA
Conclusion for Sourcing Cooling Blunt Tip:
After a thorough evaluation of potential suppliers, technical specifications, cost implications, and quality assurance measures, sourcing a cooling blunt tip requires a balanced approach that prioritizes performance, reliability, and compliance with industry standards. The ideal supplier should demonstrate proven expertise in thermal management solutions, offer consistent product quality with proper certifications, and provide technical support for integration into existing systems. Additionally, considerations such as lead times, scalability, and total cost of ownership—beyond just unit price—must be factored into the final decision. By selecting a reputable and responsive supplier, organizations can ensure efficient thermal performance, improved safety, and long-term operational efficiency in applications utilizing cooling blunt tips. Moving forward, establishing strong supplier partnerships and ongoing performance monitoring will be key to maintaining supply chain resilience and product excellence.




![[PDF] Claimant Handbook](https://www.sohoinchina.com/wp-content/uploads/2026/01/pdf-claimant-handbook-997.jpg)