Introduction: Navigating the Global Market for CIP Systems
Clean-in-Place (CIP) systems have become the backbone of sanitation in modern food, beverage, dairy, and pharmaceutical manufacturing. For operations processing thousands of liters daily, the question isn’t whether to invest in CIP technology—it’s how to select the right system for your specific production environment.
The Challenge Facing Today’s Manufacturers
Manual cleaning methods are no longer viable for competitive operations. Consider the limitations of pre-CIP manufacturing:
| Manual Cleaning Constraints | CIP Capabilities |
|—————————-|——————|
| Pipe lengths limited to 10 feet | Unlimited piping configurations |
| Tank heights capped at 8 feet | Large-scale vessel cleaning |
| Labor-intensive disassembly | Automated, validated procedures |
| Inconsistent sanitation results | Uniform, repeatable cleaning |
As regulatory requirements tighten across the USA and Europe, and production demands increase, selecting the optimal CIP system directly impacts your operational efficiency, compliance standing, and bottom line.
What This Guide Covers
This comprehensive B2B guide will walk you through:
Illustrative Image (Source: Google Search)
- System types and configurations suited to different production scales
- Critical selection criteria including flow rates, chemical compatibility, and automation levels
- Validation requirements for FDA, USDA, and EU regulatory compliance
- Total cost of ownership analysis beyond initial purchase price
- Leading manufacturers and suppliers serving North American and European markets
Whether you’re upgrading legacy equipment or specifying systems for a new facility, this guide provides the technical and commercial insights needed to make an informed procurement decision.
Article Navigation
- Top 10 Cip System Manufacturers & Suppliers List
- Introduction: Navigating the Global Market for cip system
- Understanding cip system Types and Variations
- Key Industrial Applications of cip system
- 3 Common User Pain Points for ‘cip system’ & Their Solutions
- Strategic Material Selection Guide for cip system
- In-depth Look: Manufacturing Processes and Quality Assurance for cip system
- Practical Sourcing Guide: A Step-by-Step Checklist for ‘cip system’
- Comprehensive Cost and Pricing Analysis for cip system Sourcing
- Alternatives Analysis: Comparing cip system With Other Solutions
- Essential Technical Properties and Trade Terminology for cip system
- Navigating Market Dynamics and Sourcing Trends in the cip system Sector
- Frequently Asked Questions (FAQs) for B2B Buyers of cip system
- Strategic Sourcing Conclusion and Outlook for cip system
- Important Disclaimer & Terms of Use
Top 10 Cip System Manufacturers & Suppliers List
1. Clean-In-Place (CIP) Systems Suppliers – Thomasnet
Domain: thomasnet.com
Registered: 1996 (29 years)
Introduction: Custom manufacturer of clean-in-place (CIP) systems for removing contaminations from wetted surfaces inside tanks, pipework and filling machines….
2. CIP Chemicals Companies – MarketsandMarkets
Domain: marketsandmarkets.com
Registered: 2009 (16 years)
Introduction: Also, companies operating in this market, such as BASF (Germany), Ecolab (US), DOW (US), Diversey, Inc. (US), Solvay (Belgium), Alfa Laval ( ……
3. Clean-in-Place (CIP) Systems | IPEC
Domain: ipec-inc.com
Registered: 2000 (25 years)
Introduction: IPEC is a global leader in design and fabrication of custom modular Clean-In-Place (CIP) Systems. Clean-In-Place Your Process HTST Waste Treatment…
4. CIP (Clean-In-Place) Systems – Koss Industrial
Domain: kossindustrial.com
Registered: 1997 (28 years)
Introduction: In stockCIP is a process that uses water rinses, chemicals, precise temperatures and turbulence to clean soils and bacteria from the inside surfaces of pipes, vessels….
5. Clean in Place (CIP) Solutions – Ecolab
Domain: ecolab.com
Registered: 1996 (29 years)
Introduction: Optimize your CIP process to ensure food safety and product quality with our range of CIP products, engineered solutions and technical ……
6. Whole Set CIP System – VONFO
Domain: ksvonfo.com
Registered: 2020 (5 years)
Introduction: Professional Whole Set CIP System Manufacturer. Over 15 years of CIP system design and manufacture experience. Top quality with reasonable price. Supports on ……
Understanding cip system Types and Variations
Understanding CIP System Types and Variations
Selecting the right Clean-in-Place (CIP) system configuration directly impacts operational efficiency, resource consumption, and cleaning validation outcomes. The evolution from manual cleaning methods—which historically limited tank heights to 8 feet and pipe lengths to 10 feet—to automated CIP systems has enabled modern food processing facilities to scale operations while maintaining stringent hygiene standards.

Illustrative Image (Source: Google Search)
This section examines the primary CIP system architectures available to processors, helping you match system capabilities to your specific operational requirements.
CIP System Types: Comparison Overview
| Type | Key Features | Best Applications | Pros | Cons |
|---|---|---|---|---|
| Single-Use (Single-Pass) | Fresh solutions for each cycle; no recovery | High-risk products; allergen processing; pharmaceutical | Maximum cleaning assurance; no cross-contamination risk | Higher chemical/water consumption; increased operating costs |
| Re-Use (Recovery) | Solutions recovered, stored, reconstituted | Dairy processing; beverage production; large-scale operations | Lower chemical/water costs; environmentally efficient | Requires solution monitoring; potential carryover risk |
| Multi-Tank | Dedicated tanks for each cleaning agent | Complex facilities; multi-product plants | Precise chemical control; flexible sequencing | Higher capital cost; larger footprint |
| Single-Tank | One tank with sequential solution changes | Smaller operations; single-line applications | Compact design; lower initial investment | Limited flexibility; longer cycle times |
| Portable/Mobile | Skid-mounted; moveable between locations | Multi-building facilities; temporary installations | Flexibility; serves multiple areas | Manual positioning required; capacity limitations |
Single-Use (Single-Pass) CIP Systems
Single-use systems prepare fresh cleaning solutions for each cycle, discharging them to drain after a single pass through the equipment circuit. This architecture eliminates solution degradation concerns and prevents any possibility of cross-contamination between cleaning cycles.
Operational Characteristics:
– Fresh water, detergent, and sanitiser prepared per cycle
– No solution storage or reconstitution requirements
– Simplified system design with fewer tanks and controls
Ideal Applications:
– Allergen-sensitive production environments
– Pharmaceutical and nutraceutical manufacturing
– Facilities processing high-fat or high-protein products that rapidly degrade cleaning solutions
– Operations where regulatory requirements mandate single-use protocols

Illustrative Image (Source: Google Search)
Cost Considerations:
Single-use systems typically consume 30-50% more water and chemicals compared to recovery systems. However, reduced validation complexity and elimination of solution monitoring equipment can offset these costs in appropriate applications.
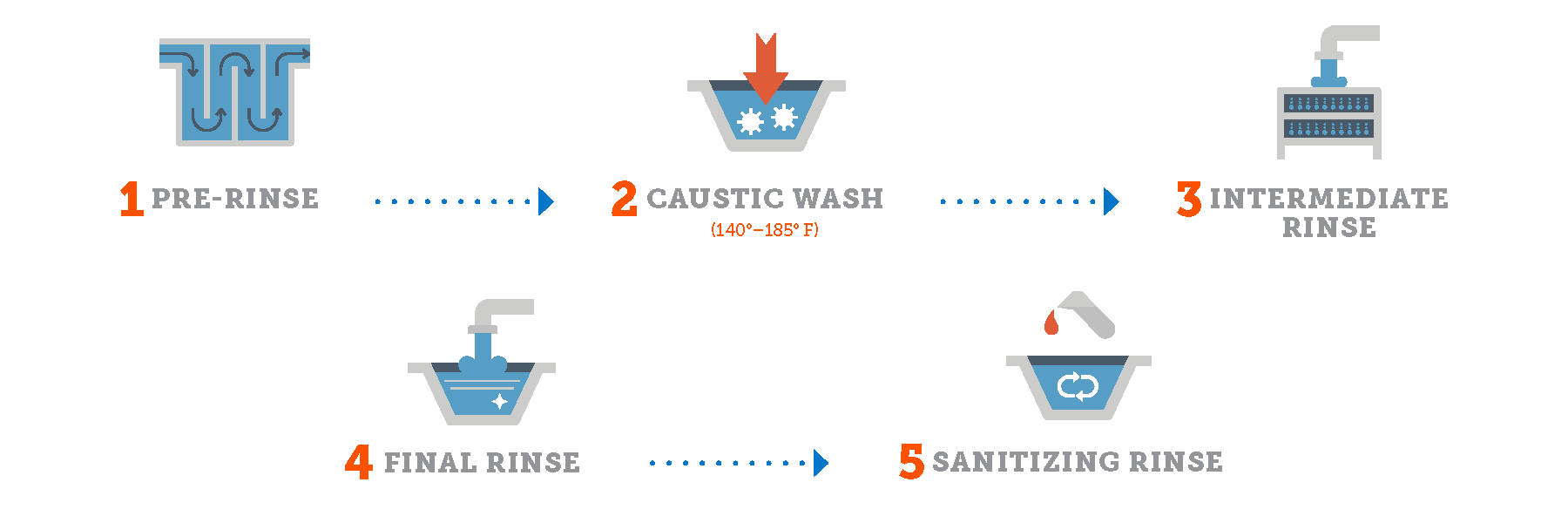
Re-Use (Recovery) CIP Systems
Recovery systems capture cleaning solutions after each cycle, store them in dedicated tanks, and reconstitute them for subsequent use. This approach—pioneered in dairy applications during the 1950s and 1960s—remains the dominant configuration in high-volume processing facilities.
Operational Characteristics:
– Solutions recovered to storage tanks after cycle completion
– Automatic titration systems restore chemical concentrations
– Temperature maintained in insulated tanks to reduce energy consumption
– Solution life typically ranges from 1-7 days depending on soil load and product type
Ideal Applications:
– Dairy processing plants (the original CIP application)
– Beverage production facilities
– Operations with multiple daily cleaning cycles
– Facilities prioritising water and chemical conservation
Illustrative Image (Source: Google Search)
Critical Success Factors:
– Continuous conductivity and temperature monitoring
– Scheduled solution replacement protocols
– Proper tank sizing to accommodate circuit volumes
– First-rinse segregation to prevent excessive soil loading
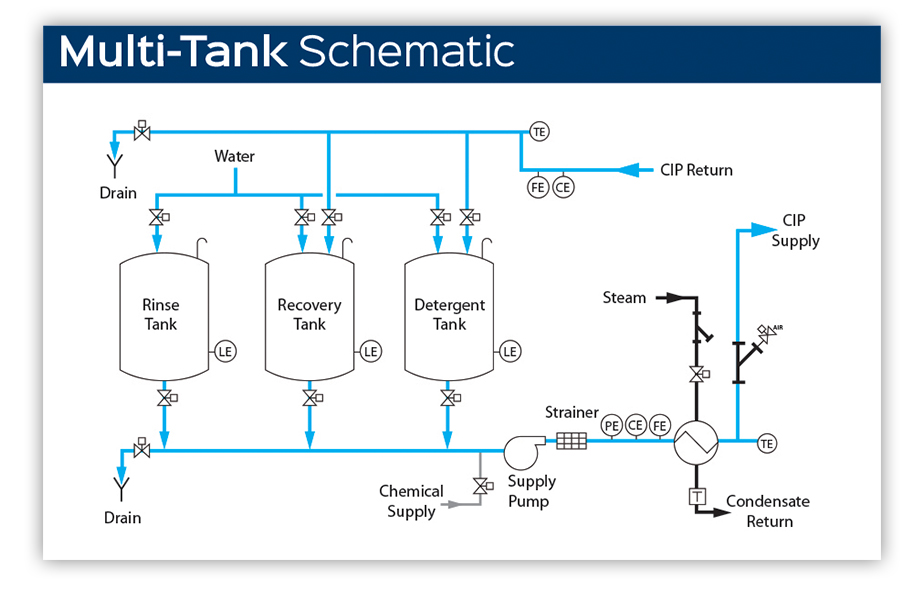
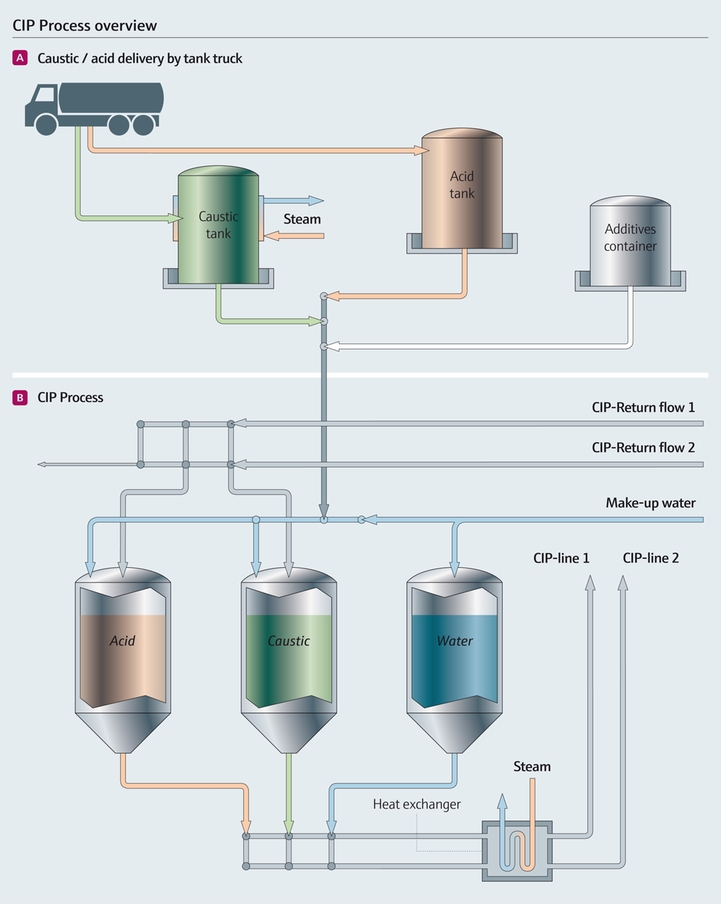
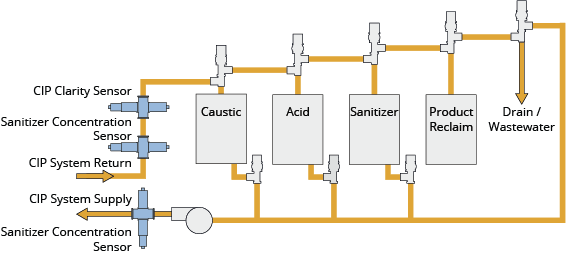
Multi-Tank CIP Systems
Multi-tank configurations dedicate separate vessels to each cleaning agent—typically including tanks for caustic solution, acid solution, fresh water supply, and recovered rinse water. This architecture provides maximum flexibility for complex cleaning sequences.
Standard Configuration:
– Caustic (alkaline) solution tank
– Acid solution tank
– Fresh water supply tank
– Recovered water tank (optional)
– Sanitiser tank (optional, or inline dosing)
Operational Characteristics:
– Independent temperature control for each solution
– Parallel solution preparation reduces cycle time
– Supports multiple circuits with different cleaning requirements
– Enables automated sequencing without manual intervention
Illustrative Image (Source: Google Search)
Ideal Applications:
– Large-scale dairy and beverage operations
– Facilities with multiple production lines requiring simultaneous cleaning
– Plants processing products with varying soil characteristics
– Operations requiring both alkaline and acid cleaning phases
Design Considerations:
Multi-tank systems require significantly more floor space and higher capital investment. However, the ability to optimise each solution independently typically delivers superior cleaning performance and faster cycle times in complex facilities.
Single-Tank CIP Systems
Single-tank systems utilise one primary vessel for solution preparation and storage, with sequential changeover between cleaning agents. This configuration suits operations with straightforward cleaning requirements and limited space.
Operational Characteristics:
– Solutions prepared and used sequentially from one tank
– Tank flushed between chemical changes
– Simplified control logic and reduced component count
– Lower installation and maintenance complexity
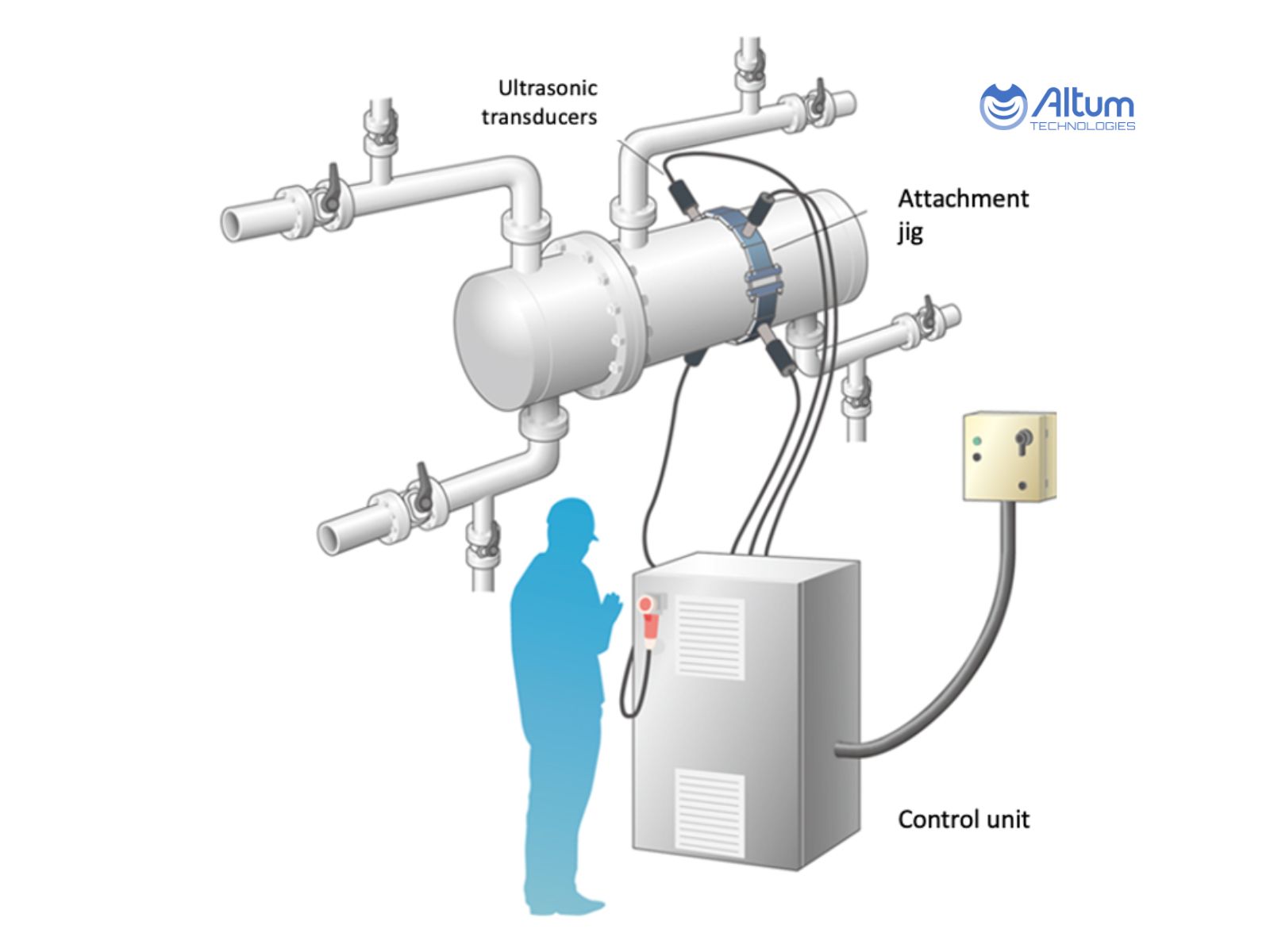
Illustrative Image (Source: Google Search)
Ideal Applications:
– Small to medium processing operations
– Single production line facilities
– Craft beverage producers
– Operations with consistent product types and soil loads
Limitations:
Sequential operation extends total cycle time compared to multi-tank systems. Additionally, thorough tank rinsing between chemical phases is essential to prevent solution contamination and ensure cleaning efficacy.
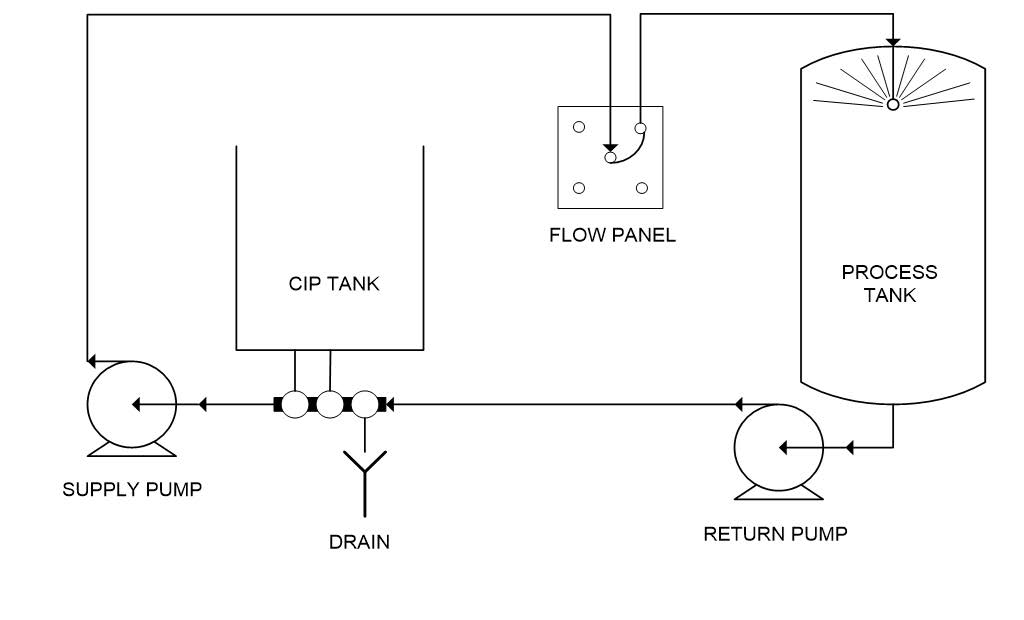
Portable (Mobile) CIP Systems
Portable CIP units mount cleaning equipment on wheeled skids or carts, enabling deployment across multiple locations within a facility. These systems address cleaning requirements where fixed installations prove impractical or cost-prohibitive.
Operational Characteristics:
– Self-contained skid with pumps, tanks, heaters, and controls
– Manual or powered mobility between cleaning locations
– Quick-connect fittings for rapid circuit attachment
– Typically single-use or limited recovery capability
Illustrative Image (Source: Google Search)
Ideal Applications:
– Multi-building processing campuses
– Facilities with infrequently cleaned equipment
– Temporary or seasonal processing operations
– Backup capacity for fixed system maintenance periods
Design Considerations:
Portable systems sacrifice some automation capability for flexibility. Ensure adequate utility connections (water, power, drain) exist at each deployment location. Tank capacity limitations may require multiple fill cycles for large equipment circuits.
Spray Device Integration Across System Types
Regardless of system architecture, effective CIP depends on proper spray device selection and positioning. As noted in CIP fundamentals, cleaning solution must contact all surfaces requiring cleaning—achieved through spray heads or flooding methods.
Common Spray Device Categories:
– Static spray balls: Fixed-pattern devices for simple tank geometries
– Rotating spray heads: Mechanical or hydraulic rotation for enhanced coverage
– Tank washing machines: High-impact devices for heavily soiled applications

Illustrative Image (Source: Google Search)
Flooded systems—where equipment fills completely with cleaning solution—typically incorporate hydraulic or mechanical agitation to ensure adequate soil removal from all surfaces.
Selection Criteria Summary
When evaluating CIP system types, consider these primary factors:
- Production volume and frequency: Higher volumes favour recovery systems
- Product characteristics: Allergen concerns may mandate single-use
- Facility layout: Distributed equipment may benefit from portable units
- Capital vs. operating cost priorities: Multi-tank systems cost more initially but often deliver lower per-cycle costs
- Validation requirements: Simpler systems ease documentation burden
- Sustainability goals: Recovery systems significantly reduce water and chemical consumption
The optimal configuration often combines elements from multiple system types—for example, a multi-tank recovery system for primary production equipment with portable single-use units for auxiliary cleaning needs.
Key Industrial Applications of cip system
Key Industrial Applications of CIP Systems
Clean-in-Place (CIP) systems have become essential infrastructure across industries where hygiene, regulatory compliance, and operational efficiency are non-negotiable. Below is a comprehensive breakdown of primary applications and their sector-specific benefits.
Industry Application Matrix
| Industry | Primary Applications | Key Equipment Cleaned |
|---|---|---|
| Dairy Processing | Milk pasteurization lines, cheese production, yogurt manufacturing | Storage tanks, heat exchangers, piping systems, homogenizers, centrifugal separators |
| Beverage Production | Brewing, soft drink bottling, juice processing, wine/spirits production | Fermentation tanks, filling lines, carbonation systems, filtration units |
| Pharmaceutical Manufacturing | API production, sterile manufacturing, bioprocessing | Reactors, bioreactors, transfer lines, filter housings, mixing vessels |
| Food Processing | Sauce production, prepared foods, confectionery, edible oils | Mixing tanks, conveyors, coating systems, storage vessels |
| Personal Care & Cosmetics | Lotions, creams, shampoos, liquid soaps | Emulsification tanks, filling equipment, transfer piping |
| Biotechnology | Cell culture production, enzyme manufacturing, fermentation | Bioreactors, chromatography systems, ultrafiltration units |
Detailed Benefits by Sector
Dairy Processing
- Regulatory alignment: Meets FDA, USDA, and EU dairy hygiene standards without manual intervention
- Production scalability: Enables storage tanks exceeding 8 feet and piping runs beyond 10 feet—impossible with manual cleaning
- Reduced contamination risk: Eliminates human contact with product-contact surfaces during cleaning cycles
- Faster turnaround: Automated cycles reduce cleaning time by 50-70% compared to disassembly methods
Beverage Production
- Flavor integrity: Prevents cross-contamination between product batches and flavor profiles
- Consistent sanitation: Validated cleaning parameters ensure repeatable results across production runs
- Extended equipment life: Controlled chemical concentrations and temperatures reduce material degradation
- Water and chemical optimization: Programmable systems minimize resource consumption per cleaning cycle
Pharmaceutical Manufacturing
- GMP compliance: Supports validation requirements for 21 CFR Part 211 and EU GMP Annex 15
- Documented traceability: Automated systems generate cleaning records required for regulatory audits
- Cross-contamination prevention: Critical for multi-product facilities producing different APIs
- Sterility assurance: Integrated sanitization steps maintain aseptic conditions in sterile manufacturing
Food Processing
- Allergen control: Validated CIP protocols demonstrate effective allergen removal between production runs
- HACCP integration: Cleaning data supports hazard analysis and critical control point documentation
- Operational flexibility: Enables rapid changeovers between different product formulations
- Labor reallocation: Frees personnel from manual cleaning tasks for higher-value production activities
Personal Care & Cosmetics
- Product quality consistency: Removes residues that could affect emulsion stability or product appearance
- Microbiological control: Prevents bacterial growth in water-based formulations
- Batch-to-batch integrity: Ensures color and fragrance accuracy across production runs
Biotechnology
- Bioburden reduction: Achieves required microbial reduction levels for sensitive biological processes
- Cell line protection: Prevents contamination that could compromise expensive cell cultures
- Validation documentation: Supports process validation for biologics manufacturing
Cross-Industry Operational Advantages
| Benefit Category | Impact |
|---|---|
| Labor Efficiency | Reduces cleaning labor requirements by 60-80% |
| Safety Improvement | Eliminates confined space entry and chemical handling exposure |
| Equipment Utilization | Increases productive uptime through faster, scheduled cleaning cycles |
| Resource Conservation | Optimizes water, chemical, and energy consumption through precise control |
| Quality Assurance | Delivers validated, repeatable cleaning outcomes with documented parameters |
| Scalability | Removes physical size constraints imposed by manual cleaning limitations |
System Validation Considerations
Regardless of industry, CIP system effectiveness depends on validated procedures that confirm cleaning solutions contact all product-contact surfaces through spray devices or flooding mechanisms. Flooded systems typically incorporate hydraulic or mechanical agitation to ensure complete soil removal. Validation protocols should address cleaning agent selection, contact time, temperature parameters, and flow rates specific to each application.
3 Common User Pain Points for ‘cip system’ & Their Solutions
3 Common User Pain Points for CIP Systems & Their Solutions
Pain Point 1: Inconsistent Cleaning Results Across Production Lines
Scenario: A mid-sized dairy processor operates multiple production lines with varying tank sizes and piping configurations. Quality control reports show inconsistent microbial counts between lines, and some batches require re-cleaning—causing production delays and increased chemical costs.
Problem: Manual cleaning methods and poorly designed CIP circuits fail to deliver uniform coverage across all equipment surfaces. Spray devices may not reach upper tank surfaces effectively, and flow rates through piping systems vary based on configuration.
Solution:
| Action | Implementation |
|——–|—————-|
| Conduct spray coverage analysis | Map all surfaces requiring cleaning and verify spray device placement |
| Standardize flow velocities | Ensure minimum 5 ft/sec flow through all piping circuits |
| Install appropriate spray devices | Match static spray balls or rotating heads to vessel geometry |
| Implement automated monitoring | Track flow rates, temperatures, and chemical concentrations in real-time |
Illustrative Image (Source: Google Search)
Pain Point 2: Excessive Water, Chemical, and Energy Consumption
Scenario: A beverage manufacturer’s CIP operations consume 40% more utilities than industry benchmarks. Extended rinse cycles and conservative chemical dosing drive up operational costs while sustainability targets remain unmet.
Problem: Legacy CIP systems often rely on fixed-time cycles rather than condition-based cleaning. Without validation data, operators default to extended cycles and excessive chemical concentrations to ensure compliance.
Solution:
– Validate actual cleaning requirements through systematic testing to establish minimum effective parameters
– Install conductivity and turbidity sensors to trigger phase transitions based on actual cleanliness, not arbitrary timers
– Implement chemical recovery systems to reclaim and reuse caustic and acid solutions
– Optimize rinse sequences using cascading water recovery between pre-rinse and final rinse stages
Pain Point 3: Lack of System Validation and Audit Readiness
Scenario: A pharmaceutical contract manufacturer faces an upcoming FDA inspection. Documentation for CIP procedures exists, but validation records are incomplete, and operators cannot demonstrate that cleaning cycles consistently achieve required cleanliness levels.

Illustrative Image (Source: Google Search)
Problem: CIP systems installed without proper validation protocols create compliance risks. Without documented evidence that cleaning procedures reliably remove product residues and microbial contamination, facilities face regulatory citations, product holds, or market withdrawals.
Solution:
| Validation Component | Required Documentation |
|———————|———————-|
| Installation Qualification (IQ) | Equipment specifications, P&IDs, spray device coverage tests |
| Operational Qualification (OQ) | Cycle parameters, alarm setpoints, sequence verification |
| Performance Qualification (PQ) | Swab/rinse sample results, microbial testing, repeatability data |
| Ongoing Monitoring | Trend analysis, deviation reports, annual revalidation schedules |
Establish a validated CIP program with documented SOPs, training records, and electronic batch records that provide complete traceability for every cleaning cycle.
Strategic Material Selection Guide for cip system
Strategic Material Selection Guide for CIP Systems
Selecting appropriate materials for Clean-in-Place (CIP) systems directly impacts operational longevity, cleaning efficacy, and total cost of ownership. Material choices must withstand repeated exposure to aggressive cleaning agents, temperature cycling, and mechanical stress while maintaining sanitary standards required in food, beverage, dairy, and pharmaceutical applications.
Illustrative Image (Source: Google Search)
Critical Factors in Material Selection
Chemical Compatibility
CIP systems routinely expose materials to:
– Caustic solutions (sodium hydroxide at 0.5-2.0% concentration)
– Acidic cleaners (phosphoric, nitric, or citric acid)
– Sanitizers (chlorine-based compounds, peracetic acid, quaternary ammonium)
– High-temperature rinse water (up to 85°C/185°F)
Materials must resist corrosion, pitting, and degradation across this chemical spectrum without leaching contaminants into product contact surfaces.
Temperature and Pressure Demands
CIP cycles involve rapid temperature fluctuations and sustained elevated pressures. Materials must maintain structural integrity through:
– Thermal expansion/contraction cycles
– Pressure spikes during pump operation
– Steam sanitization protocols (where applicable)
Surface Finish Requirements
Effective CIP depends on cleaning solutions reaching all surfaces. Material selection influences achievable surface finishes—critical for preventing biofilm formation and ensuring complete soil removal without manual intervention.
Illustrative Image (Source: Google Search)
Primary Materials for CIP System Components
Stainless Steel Grades
316L Stainless Steel
The industry standard for product contact surfaces in CIP applications. The low-carbon variant (L designation) minimizes carbide precipitation during welding, preserving corrosion resistance in heat-affected zones.
- Molybdenum content (2-3%) provides superior resistance to chloride-induced pitting
- Passivated surface forms protective chromium oxide layer
- Electropolished finishes achieve Ra ≤ 0.4 µm for pharmaceutical applications
304 Stainless Steel
Acceptable for non-product contact components where chloride exposure is limited. Lower cost than 316L but susceptible to pitting in chlorinated environments.
- Suitable for structural supports, frames, and external piping
- Not recommended for systems using chlorine-based sanitizers
Duplex Stainless Steels (2205, 2507)
Emerging option for high-stress applications requiring enhanced mechanical strength and corrosion resistance.
- Higher yield strength allows thinner wall sections
- Superior stress corrosion cracking resistance
- Higher initial cost offset by extended service life in aggressive environments
Elastomers and Sealing Materials
EPDM (Ethylene Propylene Diene Monomer)
Standard choice for CIP system gaskets and seals.

Illustrative Image (Source: Google Search)
- Excellent resistance to caustic and acidic cleaners
- Temperature range: -40°C to 150°C
- FDA-compliant formulations available
- Limited compatibility with hydrocarbon-based lubricants
Silicone
Preferred for high-temperature applications and pharmaceutical systems.
- Broader temperature tolerance than EPDM
- Superior flexibility retention after thermal cycling
- Lower chemical resistance to concentrated caustics
PTFE (Polytetrafluoroethylene)
Used where universal chemical resistance is required.
- Inert to virtually all CIP chemicals
- Higher cost than EPDM
- Requires proper compression to achieve seal integrity
FKM (Fluoroelastomer/Viton®)
Selected for applications involving ozone, chlorine dioxide, or specialty sanitizers.
- Exceptional oxidizer resistance
- Limited performance with steam and hot water
Plastics and Polymers
PVDF (Polyvinylidene Fluoride)
Used in spray devices, fittings, and instrumentation housings.
Illustrative Image (Source: Google Search)
- Broad chemical resistance spectrum
- Maintains mechanical properties at elevated temperatures
- Suitable for ultrapure water systems
Polypropylene
Cost-effective option for chemical storage tanks and non-critical piping.
- Excellent acid resistance
- Limited temperature capability (<80°C continuous)
- Not suitable for product contact surfaces
Spray Device Material Considerations
Spray balls and rotating spray heads are critical CIP components subjected to continuous chemical exposure and mechanical wear. Material selection impacts cleaning pattern consistency and maintenance frequency.
Fixed Spray Balls
Typically fabricated from 316L stainless steel with electropolished surfaces. Weld quality is critical—incomplete penetration creates crevices harboring contamination.
Rotating Spray Devices
Incorporate bearing surfaces requiring compatible material pairings:
– Ceramic/stainless combinations minimize galling
– Self-lubricating polymer bearings reduce maintenance
– Material selection affects rotational reliability and cleaning coverage

Illustrative Image (Source: Google Search)
Tank and Vessel Materials
Storage tanks and processing vessels represent significant capital investment. Material selection balances initial cost against lifecycle performance.
Fabrication Considerations:
– Weld procedures must maintain corrosion resistance
– Internal surface finish specifications (Ra values) vary by application
– Jacket materials for heating/cooling may differ from product contact surfaces
Alternative Materials for Specific Applications:
– Titanium: Extreme corrosion resistance for aggressive chemical environments
– Nickel alloys (Hastelloy): Pharmaceutical applications with stringent purity requirements
– Glass-lined steel: Chemical resistance with visual inspection capability
Piping System Materials
CIP piping must accommodate cleaning solution circulation while maintaining sanitary conditions.
Orbital Welding Requirements
316L stainless steel piping for CIP systems typically requires orbital welding to achieve consistent, crevice-free joints. Manual welding introduces variability that can compromise cleanability.
Tubing vs. Pipe Specifications
– Sanitary tubing (ASME BPE): Tighter dimensional tolerances, smoother internal surfaces
– Schedule pipe: Lower cost, acceptable for utility connections
Material Selection by System Component
| Component | Primary Material | Alternative Options | Key Selection Criteria |
|---|---|---|---|
| Product contact piping | 316L SS | Duplex SS, Hastelloy | Chemical resistance, surface finish |
| Tanks/vessels | 316L SS | 304 SS (non-chloride), glass-lined | Capacity, chemical exposure |
| Pump housings | 316L SS | 316 SS, CD4MCu | Abrasion resistance, casting quality |
| Valve bodies | 316L SS | 316 SS | Pressure rating, actuation method |
| Gaskets/seals | EPDM | Silicone, PTFE, FKM | Temperature range, chemical compatibility |
| Spray devices | 316L SS | PVDF (low-pressure) | Spray pattern requirements, wear resistance |
| Heat exchanger plates | 316L SS | Titanium, 254 SMO | Thermal conductivity, chloride exposure |
| Instrumentation housings | 316 SS | PVDF, PP | Environmental exposure, accuracy requirements |
| CIP supply tanks | 316L SS | 304 SS, PP | Chemical storage, temperature |
| Control panel enclosures | 304 SS | 316 SS (washdown areas) | Environmental rating, location |
Material Compatibility Matrix
| Material | Caustic (NaOH) | Phosphoric Acid | Nitric Acid | Chlorine Sanitizers | Peracetic Acid | Steam |
|---|---|---|---|---|---|---|
| 316L SS | Excellent | Excellent | Excellent | Good | Excellent | Excellent |
| 304 SS | Excellent | Good | Good | Poor | Good | Excellent |
| EPDM | Excellent | Excellent | Good | Good | Good | Good |
| Silicone | Good | Good | Fair | Fair | Fair | Excellent |
| PTFE | Excellent | Excellent | Excellent | Excellent | Excellent | Excellent |
| FKM | Fair | Excellent | Good | Excellent | Excellent | Poor |
| PVDF | Good | Excellent | Excellent | Excellent | Excellent | Fair |
| PP | Excellent | Excellent | Good | Fair | Good | Poor |
Ratings based on typical CIP concentrations and temperatures. Verify compatibility for specific formulations.
Economic Considerations
Total Cost of Ownership Analysis
Initial material cost represents only a fraction of lifecycle expense. Evaluate:
- Replacement frequency: Premium materials often deliver lower annualized cost
- Downtime impact: Material failures during production carry significant opportunity cost
- Maintenance requirements: Self-draining designs and appropriate material selection reduce manual intervention
- Validation costs: Material changes in pharmaceutical applications trigger revalidation
Specification Development
Detailed material specifications prevent substitution of inferior grades during fabrication. Include:
- Alloy certification requirements (mill test reports)
- Surface finish specifications with measurement methodology
- Weld procedure and inspection requirements
- Elastomer compound specifications (not just generic material type)
Regional Compliance Considerations
United States
– FDA 21 CFR compliance for food contact materials
– 3-A Sanitary Standards for dairy applications
– ASME BPE for pharmaceutical systems
European Union
– EC 1935/2004 food contact materials regulation
– EHEDG guidelines for hygienic design
– EN 10088 stainless steel specifications
Material certifications and documentation requirements vary by region. Specify compliance requirements during procurement to ensure appropriate traceability documentation accompanies delivered materials.
In-depth Look: Manufacturing Processes and Quality Assurance for cip system
In-depth Look: Manufacturing Processes and Quality Assurance for CIP Systems
Clean-in-Place (CIP) systems represent sophisticated engineering solutions that demand precision manufacturing and rigorous quality control. Understanding how these systems are built—and the standards governing their production—enables procurement teams to evaluate suppliers effectively and ensure long-term operational reliability.
Manufacturing Process Overview
CIP system manufacturing follows a structured workflow designed to meet the stringent hygiene requirements of food, beverage, dairy, and pharmaceutical applications.
Stage 1: Material Preparation
| Process Step | Description | Critical Factors |
|---|---|---|
| Material Selection | Sourcing of stainless steel (typically 304 or 316L grade) | Corrosion resistance, surface finish capability |
| Incoming Inspection | Verification of material certificates and specifications | Mill test reports, chemical composition analysis |
| Cutting & Sizing | Precision cutting of sheet metal, tubing, and structural components | Dimensional accuracy, edge quality |
| Surface Preparation | Initial cleaning and degreasing of raw materials | Contamination prevention |
316L stainless steel is preferred for pharmaceutical and aggressive chemical applications due to its superior corrosion resistance and lower carbon content, which minimizes carbide precipitation during welding.
Stage 2: Component Forming
Tank Fabrication
– Sheet rolling and forming for cylindrical vessels
– Dished end pressing for tank heads
– CNC machining for ports, fittings, and mounting points
– Internal surface finishing to achieve required Ra values (typically ≤0.8 µm for sanitary applications)
Piping and Tubing
– Orbital welding for consistent, repeatable pipe joints
– Automated bending to minimize stress points
– Electropolishing for enhanced cleanability and corrosion resistance
Spray Device Manufacturing
Spray balls and rotary spray heads—critical components referenced in CIP fundamentals—require:
– Precision drilling or laser cutting for spray orifices
– Balanced geometry for uniform coverage
– Surface finishing to prevent bacterial harborage
Stage 3: Assembly and Integration
The assembly phase brings together mechanical, electrical, and control system components:
Mechanical Assembly
– Pump installation and alignment
– Valve manifold construction
– Heat exchanger integration
– Tank and piping system connection
Instrumentation Integration
– Temperature sensors (RTDs, thermocouples)
– Conductivity probes for chemical concentration monitoring
– Flow meters and pressure transmitters
– Level sensors for tanks and vessels
Control System Installation
– PLC/HMI programming and configuration
– Wiring and cable management
– I/O testing and signal verification
Skid-Mounted Systems
Many CIP systems are delivered as pre-assembled, skid-mounted units, which offers:
– Factory-controlled assembly conditions
– Reduced on-site installation time
– Pre-commissioning and testing before shipment
Stage 4: Quality Control and Testing
Quality assurance spans the entire manufacturing process, with critical checkpoints at each stage:
In-Process Quality Checks
| Inspection Point | Method | Acceptance Criteria |
|---|---|---|
| Weld Integrity | Visual, dye penetrant, X-ray (as required) | No cracks, porosity, or incomplete fusion |
| Surface Finish | Profilometer measurement | Ra ≤ 0.8 µm (sanitary), ≤ 0.4 µm (pharmaceutical) |
| Dimensional Accuracy | CMM, calipers, gauges | Per engineering drawings ± tolerances |
| Material Traceability | Documentation review | Full traceability to mill certificates |
Final Testing Protocols
- Hydrostatic Pressure Testing: Verifies vessel and piping integrity under pressure
- Leak Testing: Confirms all connections and seals are secure
- Spray Coverage Testing: Validates that spray devices achieve complete surface contact (riboflavin testing commonly used)
- Functional Testing: Confirms pump performance, valve operation, and instrumentation accuracy
- Control System Validation: Verifies sequence logic, alarms, and safety interlocks
Quality Standards and Certifications
Reputable CIP system manufacturers adhere to internationally recognized standards:
ISO Certifications
| Standard | Scope | Relevance to CIP Systems |
|---|---|---|
| ISO 9001:2015 | Quality Management Systems | Overall manufacturing quality processes |
| ISO 14001:2015 | Environmental Management | Sustainable manufacturing practices |
| ISO 45001:2018 | Occupational Health & Safety | Worker safety during production |
Industry-Specific Standards
- 3-A Sanitary Standards: Defines hygienic design criteria for dairy and food processing equipment (USA)
- EHEDG Guidelines: European Hygienic Engineering & Design Group standards for cleanability and material selection
- ASME BPE: Bioprocessing Equipment standard for pharmaceutical and biotech applications
- FDA 21 CFR Part 11: Compliance requirements for electronic records and signatures in regulated industries
- GAMP 5: Good Automated Manufacturing Practice for control system validation
System Validation Considerations
As noted in CIP fundamentals, system validation is essential to confirm that equipment performs as intended. Manufacturers should provide:
- Factory Acceptance Testing (FAT): Documented testing performed before shipment
- Site Acceptance Testing (SAT): Verification of performance after installation
- Installation Qualification (IQ): Confirms proper installation per specifications
- Operational Qualification (OQ): Verifies operation within defined parameters
- Performance Qualification (PQ): Demonstrates consistent performance under actual operating conditions
Supplier Evaluation Criteria
When assessing CIP system manufacturers, consider:
- Documented quality management system with relevant ISO certifications
- Industry-specific compliance (3-A, EHEDG, ASME BPE as applicable)
- Weld documentation packages including welder certifications and weld logs
- Material traceability from raw material to finished product
- Validation support including IQ/OQ documentation and protocols
- After-sales support for spare parts, service, and technical assistance
Key Takeaways
- CIP system manufacturing requires specialized expertise in sanitary fabrication techniques
- Quality assurance must be integrated throughout the production process, not just at final inspection
- Compliance with ISO standards provides baseline quality assurance; industry-specific standards (3-A, EHEDG, ASME BPE) address hygienic design requirements
- Validation documentation is critical for regulated industries and should be specified during procurement
- Thorough supplier evaluation reduces risk and ensures equipment meets operational requirements
Practical Sourcing Guide: A Step-by-Step Checklist for ‘cip system’
Practical Sourcing Guide: A Step-by-Step Checklist for CIP Systems
Sourcing a Clean-in-Place (CIP) system requires systematic evaluation of technical requirements, supplier capabilities, and operational compatibility. This checklist provides a structured approach for procurement teams and plant engineers.
Phase 1: Define Internal Requirements
Application Assessment
– [ ] Identify all equipment requiring CIP (tanks, piping, heat exchangers, homogenizers, separators)
– [ ] Document current cleaning protocols and pain points
– [ ] Determine cleaning method needed: spray-based, flooded, or combination
– [ ] Calculate total surface area and system volume to be cleaned
– [ ] Map piping runs and identify maximum distances from CIP supply
Operational Parameters
– [ ] Define production schedule and available cleaning windows
– [ ] Establish required cleaning cycle times
– [ ] Identify soil types (proteins, fats, minerals, biofilms)
– [ ] Document temperature and pressure requirements
– [ ] Specify water quality and availability
Compliance Requirements
– [ ] Identify applicable regulatory standards (FDA, USDA, EHEDG, 3-A Sanitary Standards)
– [ ] Document validation requirements for your industry
– [ ] Determine record-keeping and traceability needs
Phase 2: Technical Specification Development
| Specification Area | Key Considerations |
|---|---|
| System Capacity | Flow rates, tank volumes, simultaneous circuit capability |
| Spray Devices | Static spray balls, rotating heads, orbital cleaners |
| Cleaning Agents | Caustic, acid, sanitizer compatibility; concentration control |
| Automation Level | Manual, semi-automated, fully automated PLC control |
| Instrumentation | Temperature, flow, conductivity, pressure sensors |
| Integration | SCADA compatibility, existing plant control systems |
Phase 3: Supplier Qualification
Initial Screening
– [ ] Request capability statements from minimum 3-5 suppliers
– [ ] Verify industry experience (dairy, beverage, pharmaceutical, food processing)
– [ ] Confirm geographic coverage and service network in USA/Europe
– [ ] Check references from comparable installations
Technical Evaluation
– [ ] Review proposed spray device selection and coverage calculations
– [ ] Assess system validation methodology
– [ ] Evaluate chemical handling and dosing systems
– [ ] Examine automation and control architecture
– [ ] Request P&IDs and equipment specifications
Commercial Assessment
– [ ] Compare total cost of ownership (equipment, installation, chemicals, utilities)
– [ ] Review warranty terms and conditions
– [ ] Evaluate spare parts availability and lead times
– [ ] Assess training and commissioning support
Phase 4: Request for Proposal (RFP) Essentials
Include the following in your RFP:
- Site conditions – utility availability, space constraints, ambient conditions
- Performance criteria – cleaning efficacy targets, cycle time limits
- Validation requirements – documentation, testing protocols, acceptance criteria
- Project timeline – design, fabrication, installation, commissioning milestones
- Support expectations – training, spare parts, ongoing service agreements
Phase 5: Final Selection and Contracting
- [ ] Conduct site visits to reference installations
- [ ] Perform technical clarification meetings with shortlisted suppliers
- [ ] Negotiate performance guarantees and penalties
- [ ] Define acceptance testing procedures
- [ ] Establish system validation protocols before contract execution
- [ ] Confirm post-installation support and service level agreements
Key Questions for Supplier Discussions
- How do you validate uniform cleaning coverage for complex geometries?
- What instrumentation do you recommend for continuous monitoring?
- How does your system minimize water, chemical, and energy consumption?
- What is your approach to system validation documentation?
- What training do you provide for operations and maintenance personnel?
Next Step: Develop a weighted scoring matrix based on your priority requirements before issuing RFPs to ensure objective supplier comparison.
Comprehensive Cost and Pricing Analysis for cip system Sourcing
Comprehensive Cost and Pricing Analysis for CIP System Sourcing
Investing in Clean-In-Place (CIP) systems requires careful financial planning to balance operational efficiency with budget constraints. This analysis provides a detailed breakdown of costs and actionable strategies to optimize your procurement investment.
Total Cost of Ownership Framework
CIP system costs extend far beyond the initial purchase price. A comprehensive TCO analysis should account for acquisition, installation, operation, and maintenance over a 10-15 year lifecycle.
Materials Cost Breakdown
| Component Category | Cost Range (USD) | % of Total System Cost |
|---|---|---|
| Tanks & Vessels | $15,000 – $75,000 | 15-20% |
| Pumps & Motors | $8,000 – $45,000 | 10-15% |
| Spray Devices & Nozzles | $2,000 – $15,000 | 3-5% |
| Piping & Valves | $10,000 – $50,000 | 12-18% |
| Heat Exchangers | $12,000 – $60,000 | 10-15% |
| Instrumentation & Sensors | $8,000 – $35,000 | 8-12% |
| Control Systems (PLC/HMI) | $15,000 – $80,000 | 15-25% |
| Structural Framework | $5,000 – $25,000 | 5-8% |
Material Selection Impact:
– 304 Stainless Steel: Standard grade, cost-effective for most applications
– 316L Stainless Steel: 20-30% premium, required for corrosive environments
– Specialty Alloys: 50-100% premium for pharmaceutical-grade applications
Labor Costs
Engineering & Design
| Service | Cost Range (USD) |
|---|---|
| Process Engineering | $10,000 – $40,000 |
| System Design & P&ID Development | $8,000 – $25,000 |
| Validation Documentation | $5,000 – $30,000 |
| Project Management | $12,000 – $35,000 |
Installation Labor
| Phase | Cost Range (USD) | Duration |
|---|---|---|
| Mechanical Installation | $20,000 – $80,000 | 2-6 weeks |
| Electrical & Controls | $15,000 – $50,000 | 1-4 weeks |
| Commissioning | $8,000 – $25,000 | 1-2 weeks |
| Operator Training | $3,000 – $12,000 | 3-5 days |
Regional Labor Rate Variations:
– USA (Midwest): $65-95/hour
– USA (Coastal): $85-125/hour
– Western Europe: €70-110/hour
– Eastern Europe: €35-60/hour
Logistics Costs
| Logistics Component | Cost Factors |
|---|---|
| Domestic Freight (USA) | $2,000 – $15,000 depending on distance and system size |
| International Shipping | $8,000 – $35,000 (container rates, customs, duties) |
| Rigging & Crane Services | $3,000 – $12,000 |
| Storage & Warehousing | $500 – $2,000/month if staging required |
| Insurance (Transit) | 0.5-1.5% of equipment value |
Import Duty Considerations:
– USA: 0-6.5% depending on component classification
– EU: 0-4.5% with potential exemptions under trade agreements
Ongoing Operational Costs (Annual)
| Category | Annual Cost Range (USD) |
|---|---|
| Cleaning Chemicals (Caustic, Acid, Sanitizers) | $8,000 – $45,000 |
| Water Consumption | $3,000 – $20,000 |
| Energy (Heating, Pumping) | $5,000 – $30,000 |
| Replacement Parts & Consumables | $4,000 – $15,000 |
| Preventive Maintenance Labor | $6,000 – $20,000 |
| Validation & Compliance Testing | $3,000 – $15,000 |
Cost-Saving Strategies
Procurement Optimization
- Consolidate Vendors: Negotiate volume discounts by sourcing multiple components from single suppliers
- Standardize Specifications: Reduce engineering costs by using pre-engineered modular systems
- Competitive Bidding: Obtain minimum 3-5 quotes; leverage bids to negotiate 10-15% reductions
- Off-Peak Ordering: Schedule procurement during Q1 when manufacturers offer incentives
Design Efficiency
- Right-Size Systems: Avoid over-engineering; match CIP capacity to actual production requirements
- Circuit Optimization: Minimize piping runs and reduce the number of spray devices needed
- Heat Recovery Integration: Reduce energy costs by 20-35% through heat exchanger optimization
Installation Savings
- Pre-Fabrication: Request skid-mounted systems to reduce on-site labor by 30-40%
- Local Contractors: Utilize regional installation teams to minimize travel expenses
- Phased Implementation: Stage installation during planned downtime to avoid overtime premiums
Operational Cost Reduction
- Chemical Recovery Systems: Reclaim and reuse cleaning solutions to reduce chemical costs by 40-60%
- Water Recycling: Implement final rinse water recovery for pre-rinse cycles
- Automated Monitoring: Invest in conductivity and turbidity sensors to optimize cycle times and prevent over-cleaning
- Preventive Maintenance Programs: Reduce unplanned downtime and extend equipment life by 25-40%
Pricing Models Comparison
| Procurement Model | Advantages | Disadvantages |
|---|---|---|
| Turnkey (EPC) | Single-source accountability, reduced coordination | 15-25% cost premium |
| Design-Bid-Build | Competitive pricing, flexibility | Longer timeline, coordination burden |
| Equipment-Only Purchase | Lowest capital cost | Requires internal engineering resources |
| Lease/Rental | Preserves capital, includes maintenance | Higher long-term cost, limited customization |
Budget Planning Summary
| System Scale | Typical Total Investment (USD) |
|---|---|
| Small (Single Circuit) | $75,000 – $150,000 |
| Medium (Multi-Circuit) | $200,000 – $500,000 |
| Large (Facility-Wide) | $750,000 – $2,000,000+ |
Key Takeaway: Allocate 15-20% contingency for unforeseen requirements, particularly for validation, facility modifications, and utility upgrades. Early investment in proper system design and validation documentation yields significant long-term savings through reduced operational costs and regulatory compliance efficiency.
Alternatives Analysis: Comparing cip system With Other Solutions
Alternatives Analysis: Comparing CIP Systems With Other Solutions
When evaluating cleaning methodologies for food, beverage, pharmaceutical, and industrial processing operations, decision-makers must weigh operational efficiency, compliance requirements, and total cost of ownership. This analysis examines CIP systems against two primary alternatives: manual cleaning (Clean-Out-of-Place/COP) and semi-automated hybrid systems.
Comparison Overview
| Factor | CIP Systems | Manual Cleaning (COP) | Semi-Automated Hybrid |
|---|---|---|---|
| Labor Requirements | Minimal operator intervention | High—requires disassembly and hand scrubbing | Moderate—partial automation with manual steps |
| Cleaning Consistency | Validated, repeatable procedures | Variable—dependent on operator technique | Improved over manual, less consistent than CIP |
| Equipment Size Limitations | Virtually unlimited | Tanks ≤8 ft height; pipes ≤10 ft length | Moderate constraints |
| Downtime | Reduced—no disassembly required | Extended—full disassembly and reassembly | Moderate |
| Initial Capital Investment | Higher | Lower | Medium |
| Regulatory Compliance | Easier validation and documentation | Challenging to validate consistently | Partial validation capability |
| Risk of Contamination | Lower—closed system | Higher—exposure during handling | Moderate |
| Suitable Applications | Tanks, piping, heat exchangers, homogenizers, centrifugal separators | Small components, complex geometries | Mid-scale operations, transitional facilities |
Detailed Analysis
CIP Systems
CIP technology emerged from necessity during World War II when dairy operations needed to clean fragile Pyrex glass tubing without disassembly. By the mid-1960s, CIP became standard in dairy plants and subsequently expanded to pharmaceutical applications in the late 1970s.
Key advantages:
– Automated delivery of cleaning agents via spray devices or flooding with hydraulic/mechanical agitation
– Eliminates physical handling limitations that historically constrained equipment dimensions
– Supports continuous production schedules with predictable cleaning cycles
– Provides documented, validated cleaning procedures essential for FDA, USDA, and EU regulatory compliance
Manual Cleaning (COP)
Traditional manual methods remain viable for specific scenarios:
– Operations with limited capital budgets
– Facilities processing low-risk products with infrequent changeovers
– Equipment with complex internal geometries unsuitable for spray coverage
– Small-batch artisan producers where equipment scale remains manageable
Primary limitations include inconsistent results dependent on individual operator performance, extended downtime during disassembly/reassembly cycles, and difficulty meeting stringent validation requirements for regulated industries.
Semi-Automated Hybrid Systems
Hybrid approaches combine automated chemical delivery with manual intervention points. These systems suit operations transitioning toward full automation or facilities where certain equipment components require manual attention while primary vessels utilize automated cleaning.
Selection Criteria
Choose CIP systems when:
– Operating large-scale processing equipment
– Regulatory validation is mandatory
– Labor costs or availability present challenges
– Production schedules demand minimal cleaning downtime
Choose manual cleaning when:
– Capital constraints prohibit automation investment
– Equipment scale remains within manual handling limits
– Product risk profiles permit variable cleaning outcomes
Choose hybrid systems when:
– Phased automation implementation aligns with capital planning
– Mixed equipment types require different cleaning approaches
– Operational flexibility outweighs maximum efficiency gains
Essential Technical Properties and Trade Terminology for cip system
Essential Technical Properties and Trade Terminology for CIP Systems
Critical Technical Properties
Understanding CIP system specifications requires familiarity with key performance parameters that directly impact cleaning efficacy and operational efficiency.
Core Performance Parameters
| Property | Description | Typical Range |
|---|---|---|
| Flow Rate | Velocity of cleaning solution through piping | 1.5–3.0 m/s (5–10 ft/s) |
| Temperature | Operating temperature for cleaning cycles | 60–85°C (140–185°F) |
| Concentration | Chemical agent dilution ratio | 0.5–3.0% by volume |
| Contact Time | Duration solution contacts surfaces | 10–30 minutes per cycle |
| Pressure | System operating pressure | 1.5–3.5 bar (22–51 psi) |
| Turbidity | Rinse water clarity measurement | <5 NTU for final rinse |
Spray Device Specifications
- Spray Coverage: 360° surface contact capability
- Impact Force: Measured in N/m² at target surface
- Pattern Uniformity: Distribution consistency across vessel surfaces
- Shadow-Free Design: Elimination of uncleaned zones
Standard Trade Terminology
Commercial Terms
| Term | Definition |
|---|---|
| MOQ | Minimum Order Quantity—lowest unit count per purchase order |
| OEM | Original Equipment Manufacturer—systems built for rebranding |
| Turnkey System | Complete installation including commissioning and validation |
| Skid-Mounted | Pre-assembled, factory-tested unit on transportable frame |
| Modular Design | Scalable configuration allowing circuit additions |
Technical Abbreviations
- CIP: Clean-In-Place
- SIP: Sterilize-In-Place (often combined as CIP/SIP)
- PLC: Programmable Logic Controller
- HMI: Human-Machine Interface
- FAT: Factory Acceptance Testing
- SAT: Site Acceptance Testing
- IQ/OQ/PQ: Installation/Operational/Performance Qualification
System Configuration Terms
- Single-Use System: Fresh solution per cycle, discharged after use
- Re-Use System: Solution recovered and recirculated for multiple cycles
- Multi-Tank System: Separate vessels for caustic, acid, and rinse water
- Eductor System: Venturi-based chemical injection method
Validation and Compliance Terminology
| Term | Application |
|---|---|
| Validated Procedures | Documented, reproducible cleaning protocols meeting regulatory standards |
| 3-A Sanitary Standards | North American hygienic equipment certification |
| EHEDG Guidelines | European Hygienic Engineering & Design Group compliance |
| ATP Testing | Adenosine Triphosphate swab testing for surface cleanliness verification |
| Conductivity Monitoring | Real-time chemical concentration measurement |
Procurement Considerations
When specifying CIP systems, request documentation for:
- P&ID (Piping and Instrumentation Diagrams)
- Material Certificates (316L stainless steel verification)
- Surface Finish Specifications (Ra values, typically ≤0.8 µm)
- Cycle Recipes (programmable cleaning sequences)
- Data Logging Capabilities (21 CFR Part 11 compliance where applicable)
Navigating Market Dynamics and Sourcing Trends in the cip system Sector
Navigating Market Dynamics and Sourcing Trends in the CIP System Sector
Historical Evolution and Market Context
The Clean-in-Place (CIP) sector has undergone substantial transformation since its inception. The first automated CIP system was installed in a family-operated dairy in 1953, driven by necessity—World War II metal shortages forced dairies to adopt fragile Pyrex glass tubing that couldn’t withstand manual disassembly cleaning. By the mid-1960s, CIP had become standard in dairy operations, subsequently expanding into non-dairy food processing (1960s) and pharmaceutical manufacturing (late 1970s).
This evolution fundamentally changed equipment design possibilities. Prior to CIP:
– Pipe lengths were limited to approximately 10 feet due to manual handling constraints
– Storage tanks could not exceed 8 feet in height (manual scrubbing limitations)
– Labor-intensive disassembly/reassembly cycles constrained production throughput
Current Market Dynamics
| Market Driver | Impact on Sourcing Decisions |
|---|---|
| Regulatory compliance (FDA, EHEDG) | Preference for validated, documented systems |
| Labor cost escalation | Increased automation investment justification |
| Production scale expansion | Demand for modular, scalable CIP architectures |
| Cross-contamination concerns | Single-use vs. multi-use system evaluations |
Key Sourcing Considerations
System Configuration Options:
– Spray device systems — optimal for tanks, vessels, and accessible surfaces
– Flooded systems — required for complex geometries; utilize hydraulic or mechanical agitation
– Hybrid configurations — increasingly specified for facilities with diverse equipment profiles
Equipment Categories Commonly Requiring CIP Integration:
– Tanks and piping systems
– Filter housings and heat exchangers
– Homogenizers and centrifugal separators
– Conveyors and ductwork
Sustainability Trends Shaping Procurement
Sustainability has become a non-negotiable evaluation criterion for B2B buyers in both USA and European markets:
- Water consumption optimization — Closed-loop systems and water recovery technologies
- Chemical reduction initiatives — Concentrated cleaning agents, enzymatic alternatives
- Energy efficiency — Heat recovery from CIP cycles, optimized heating protocols
- Validation documentation — Demonstrable environmental impact metrics increasingly required in RFPs
Strategic Sourcing Recommendations
- Prioritize suppliers offering comprehensive system validation support
- Evaluate total cost of ownership, including water, chemical, and energy consumption
- Confirm compatibility with existing equipment profiles (tanks, piping diameter, heat exchanger types)
- Assess supplier capability for regulatory documentation aligned with target market requirements (FDA for USA, EHEDG standards for European operations)
Frequently Asked Questions (FAQs) for B2B Buyers of cip system
Frequently Asked Questions (FAQs) for B2B Buyers of CIP Systems
1. What is a Clean-In-Place (CIP) system and how does it work?
A CIP system is an automated cleaning method for food-processing equipment that eliminates the need for disassembly. The system circulates cleaning solutions through equipment using spray devices or flooding techniques, with agitation provided by hydraulic or mechanical means. CIP enables thorough, validated cleaning of tanks, piping systems, heat exchangers, homogenizers, centrifugal separators, and other processing equipment.
2. What types of equipment can be cleaned using CIP systems?
CIP systems are suitable for equipment where cleaning solutions can contact all surfaces requiring sanitation. Common applications include:
| Equipment Type | Application |
|---|---|
| Tanks | Product storage and processing vessels |
| Piping systems | Transfer lines and distribution networks |
| Heat exchangers | Thermal processing equipment |
| Filter housings | Filtration systems |
| Homogenizers | Product consistency equipment |
| Centrifugal separators | Separation processes |
| Conveyors | Material handling systems |
| Ductwork | Air handling and ventilation |
3. What are the primary business advantages of CIP over manual cleaning?
CIP systems deliver measurable operational benefits:
- Increased equipment capacity: Tanks can exceed 8-foot height limitations; piping systems can extend beyond 10-foot lengths
- Reduced labor costs: Automated processes eliminate manual scrubbing and disassembly
- Consistent cleaning quality: Validated procedures ensure uniform results every cycle
- Decreased downtime: Faster cleaning cycles increase production availability
- Improved worker safety: Reduces personnel exposure to cleaning chemicals and confined spaces
4. What industries benefit most from CIP system implementation?
CIP technology originated in dairy processing and has expanded across multiple sectors:
- Dairy and beverage processing
- Pharmaceutical manufacturing
- Brewery and winery operations
- Food and confectionery production
- Cosmetics manufacturing
- Biotechnology facilities
5. What cleaning agents are used in CIP systems?
CIP systems utilize specialized cleaning agents selected based on soil type, equipment materials, and regulatory requirements. Standard cleaning protocols typically include:
- Alkaline detergents: Remove organic soils (fats, proteins)
- Acid cleaners: Eliminate mineral deposits and scale
- Sanitizers: Achieve microbial reduction after cleaning
- Rinse water: Remove chemical residues between stages
Chemical selection should align with your specific product residues and equipment metallurgy.
6. How is CIP system effectiveness validated?
System validation ensures consistent cleaning performance through documented procedures that verify:
- Cleaning solution concentration and temperature parameters
- Contact time and flow rates for all circuits
- Coverage verification for spray devices
- Residue testing protocols (visual, chemical, microbiological)
- Documentation and traceability requirements
Validation protocols should meet applicable regulatory standards for your industry (FDA, USDA, 3-A Sanitary Standards).
7. What are the key components of a CIP system?
A complete CIP system typically includes:
| Component | Function |
|---|---|
| CIP supply tank(s) | Store cleaning and rinse solutions |
| Supply pump | Circulate solutions through equipment |
| Heat exchanger | Maintain solution temperature |
| Spray devices | Distribute solutions within vessels |
| Instrumentation | Monitor flow, temperature, concentration |
| Control system | Automate cleaning sequences |
| Return system | Recover solutions for reuse |
8. What factors should B2B buyers evaluate when selecting a CIP system?
Key procurement considerations include:
- Capacity requirements: Number of circuits, simultaneous cleaning needs
- Chemical compatibility: Materials of construction for your cleaning agents
- Automation level: Single-use vs. reuse systems; manual vs. fully automated
- Integration capability: Compatibility with existing equipment and control systems
- Validation support: Documentation packages for regulatory compliance
- Total cost of ownership: Water, chemical, energy consumption; maintenance requirements
- Supplier expertise: Industry experience, technical support, spare parts availability
Request detailed specifications and reference installations from vendors operating in your specific industry segment.
Strategic Sourcing Conclusion and Outlook for cip system
Strategic Sourcing Conclusion: CIP System Investment Value
Clean-in-Place systems represent a foundational investment in operational efficiency, regulatory compliance, and long-term cost reduction for food, dairy, beverage, and pharmaceutical processors.
Key Value Drivers
| Factor | Business Impact |
|---|---|
| Labor Reduction | Eliminates manual disassembly and hand-cleaning requirements |
| Equipment Scalability | Enables larger tanks and extended piping systems previously impossible with manual methods |
| Consistency | Delivers uniform, validated cleaning cycles that meet regulatory standards |
| Uptime | Reduces cleaning downtime versus traditional disassembly methods |
Sourcing Priorities
When evaluating CIP system suppliers, prioritize:
- Validation documentation supporting your regulatory environment (FDA, USDA, EU standards)
- Spray device engineering matched to your equipment geometry
- Chemical compatibility with your specific cleaning agents
- Integration capability with existing process control systems
Market Outlook
CIP technology continues advancing toward greater automation, reduced water and chemical consumption, and enhanced monitoring capabilities. Processors investing in modern CIP infrastructure position themselves for stricter hygiene regulations and sustainability requirements emerging across North American and European markets.
Bottom line: Strategic CIP sourcing directly impacts product safety, operational costs, and competitive positioning in regulated manufacturing environments.
Important Disclaimer & Terms of Use
⚠️ Important Disclaimer
The information provided is for informational purposes only. B2B buyers must conduct their own due diligence.