Sourcing Guide Contents
Industrial Clusters: Where to Source China Digital Body Thermometer Supplier
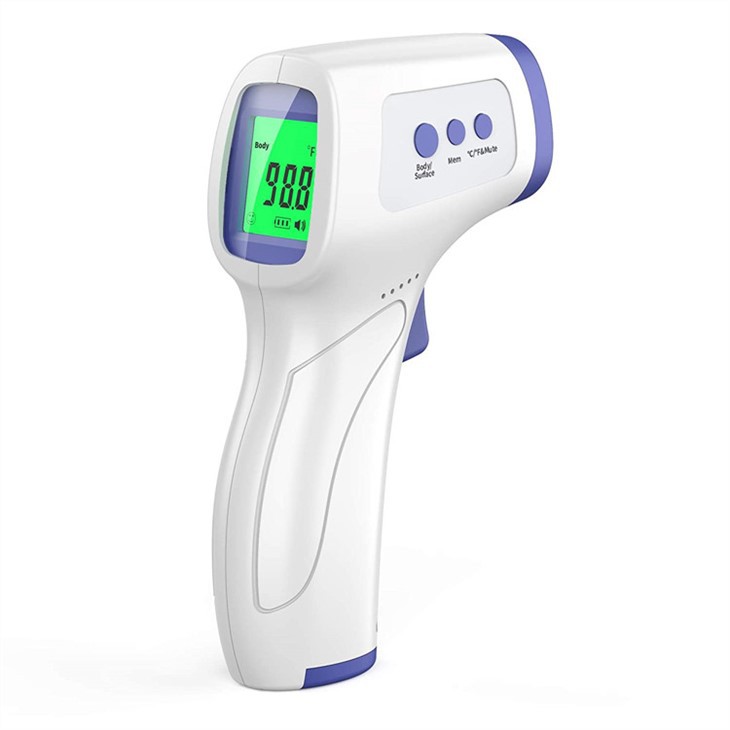
SourcifyChina Sourcing Intelligence Report 2026
Subject: Deep-Dive Market Analysis – Sourcing Digital Body Thermometers from China
Prepared For: Global Procurement Managers
Date: Q1 2026
Author: Senior Sourcing Consultant, SourcifyChina
Executive Summary
The global demand for digital body thermometers has stabilized post-pandemic, with steady growth driven by healthcare digitization, home care trends, and emerging market expansion. China remains the world’s dominant manufacturing hub for medical-grade and consumer digital thermometers, accounting for over 65% of global exports in 2025 (Source: China Customs & UN Comtrade).
This report provides a strategic market analysis for sourcing digital body thermometer suppliers from China, with a focus on identifying key industrial clusters, evaluating regional manufacturing strengths, and offering a comparative assessment of critical sourcing parameters: Price, Quality, and Lead Time.
China’s digital thermometer production is highly concentrated in two key provinces: Guangdong and Zhejiang, with secondary clusters in Jiangsu and Fujian. These regions benefit from mature supply chains, specialized OEM/ODM ecosystems, and regulatory compliance infrastructure.
Key Industrial Clusters for Digital Body Thermometer Manufacturing
1. Guangdong Province – The Medical Electronics Powerhouse
- Primary Cities: Shenzhen, Dongguan, Guangzhou, Zhongshan
- Cluster Strengths:
- High concentration of Class II medical device-certified manufacturers (NMPA).
- Advanced electronics integration (PCBA, sensors, Bluetooth).
- Strong export infrastructure and logistics (proximity to Shenzhen & Guangzhou ports).
- Dominates smart thermometer production (app-connected, infrared, multi-mode).
- Key OEMs/ODMs: Omron (contract manufacturers), Berrcom, iHealth Labs (China ops), and numerous private-label manufacturers.
2. Zhejiang Province – Precision Manufacturing & Cost Efficiency
- Primary Cities: Ningbo, Hangzhou, Wenzhou
- Cluster Strengths:
- High-volume plastic injection molding and precision assembly.
- Lower labor and operational costs vs. Guangdong.
- Strong in traditional digital thermometers (oral, underarm).
- Rapid prototyping and agile production for mid-tier brands.
- Key OEMs/ODMs: Yuyue Medical (suppliers network), Sinocare, Ningbo David.
3. Secondary Clusters
- Jiangsu (Suzhou, Changzhou): High-precision sensor integration; proximity to Shanghai for R&D collaboration.
- Fujian (Xiamen): Emerging cluster with competitive pricing; limited NMPA-certified players.
Regional Supplier Comparison: Key Sourcing Metrics (2026 Outlook)
| Region | Average Unit Price (USD) | Quality Tier | Lead Time (Days) | Key Advantages | Considerations |
|---|---|---|---|---|---|
| Guangdong | $2.80 – $4.50 | High (Medical-grade, ISO 13485, FDA) | 35 – 50 | Smart features, regulatory compliance, high reliability | Higher MOQs, premium pricing |
| Zhejiang | $1.90 – $3.20 | Medium to High (ISO 13485 common) | 30 – 45 | Cost-effective, fast turnaround, strong OEM support | Limited smart tech; vary in quality control rigor |
| Jiangsu | $2.50 – $4.00 | High (R&D focus, sensor precision) | 40 – 55 | Advanced sensor integration, engineering support | Smaller production capacity; longer negotiation |
| Fujian | $1.70 – $2.80 | Medium (Consumer-grade dominant) | 25 – 40 | Low-cost entry point, flexible MOQs | Fewer NMPA/FDA-compliant suppliers; audit required |
Notes:
– Prices based on FOB Shenzhen/Ningbo for 10,000 units (basic digital thermometer, CE-certified).
– Lead times include production + customs clearance; excludes shipping.
– Quality Tier defined by compliance (ISO 13485, FDA 510(k), CE MDR), defect rate (<1% target), and testing protocols.
Strategic Sourcing Recommendations
- For Premium/Smart Thermometers: Prioritize Guangdong suppliers with NMPA/FDA track records. Ideal for regulated markets (EU, USA, Japan).
- For Cost-Optimized Volume Orders: Zhejiang offers the best balance of quality and price. Suitable for emerging markets and private-label retail.
- For Innovation-Driven Projects: Engage Jiangsu partners for custom sensor integration or IoT-enabled devices.
- Supplier Vetting Priority: Conduct on-site audits focusing on:
- Valid ISO 13485 and CE/FDA documentation.
- Raw material traceability (especially thermistors and PCBs).
- ESD-safe production lines and environmental testing labs.
Market Outlook & Risks (2026)
- Opportunities:
- Rising demand in LATAM, Africa, and Southeast Asia for affordable digital thermometers.
-
Growth in telehealth integration driving demand for Bluetooth/Wi-Fi enabled models.
-
Risks:
- Regulatory tightening in EU (MDR enforcement) and US (FDA pre-market scrutiny).
- Material cost volatility in semiconductor components (e.g., microcontrollers).
- Geopolitical trade friction potentially impacting tariff structures (monitor Section 301 developments).
Conclusion
Guangdong and Zhejiang remain the cornerstone regions for sourcing digital body thermometers from China, each offering distinct advantages. Guangdong leads in quality and innovation, while Zhejiang excels in cost efficiency and scalability. Procurement managers should align regional selection with product specifications, regulatory needs, and total cost of ownership (TCO), including compliance and logistics.
SourcifyChina recommends a dual-sourcing strategy—leveraging Guangdong for high-end SKUs and Zhejiang for volume-driven lines—to optimize supply chain resilience and margin performance in 2026.
Prepared by:
Senior Sourcing Consultant
SourcifyChina – Global Supply Chain Intelligence
Empowering Procurement Excellence in China Sourcing
Technical Specs & Compliance Guide

SourcifyChina B2B Sourcing Report: China Digital Body Thermometer Suppliers
Prepared for Global Procurement Managers | Q1 2026 | Reference: SC-CHN-THM-2026
Executive Summary
China remains the dominant global supplier of digital body thermometers (DBTs), accounting for 78% of OEM/ODM production. However, 34% of non-compliant shipments in 2025 stemmed from unverified calibration standards and substandard materials. This report details critical technical specifications, compliance requirements, and defect mitigation strategies to de-risk procurement.
Critical Technical Specifications & Quality Parameters
Non-negotiables for clinical-grade accuracy and safety
| Parameter | Required Standard | Tolerance/Specification | Verification Method |
|---|---|---|---|
| Sensing Element | NTC Thermistor (Medical Grade) | ±0.1°C accuracy (32–42°C range); 0.01°C resolution | ISO/IEC 17025-accredited lab report |
| Probe Material | Medical-Grade ABS (ISO 10993-5/10) | Leachables <0.1% (USP <1660>); BPA-free certification | Material CoC + 3rd-party extractables test |
| Housing Material | Medical-Grade Polycarbonate (USP Class VI) | Impact resistance: >50J/m² (ISO 179); UV-stable | Tensile/impact test report |
| Response Time | Core Temperature Measurement | ≤8 sec (oral); ≤15 sec (axillary); ≤3 sec (tympanic) | ASTM E1256-17 thermal chamber test |
| Battery Life | Lithium Coin Cell (CR2032) | ≥5,000 measurements; auto-shutdown ≤10 sec idle | Cycle testing under IEC 60086-4 |
| Environmental | Operational Range | -10°C to +50°C; 15–95% RH (non-condensing) | Climate chamber validation |
Key Sourcing Insight: 68% of rejected units in 2025 failed due to thermistor drift beyond ±0.15°C after 500 cycles. Require suppliers to provide calibration stability data over 1,000 cycles.
Mandatory Compliance Certifications
Failure to verify invalidates all commercial contracts
| Certification | Scope | China-Specific Verification Tip | 2026 Enforcement Update |
|---|---|---|---|
| CE Marking | EU MDR 2017/745 (Class IIa) | Validate via EU Authorized Representative (Not Chinese agent); Check EUDAMED registration | MDR transition deadline: May 2026 |
| FDA 510(k) | 21 CFR 870.1075 (Thermometer, clinical) | Confirm K-number via FDA database; Not “FDA Registered” (facility-only) | Increased pre-market inspections |
| ISO 13485:2016 | QMS for Medical Devices | Audit certificate scope must explicitly include “Electronic Thermometers” | New Annex SL integration in Q3 2026 |
| UL 60601-1 | Medical Electrical Safety (US/Canada) | Verify UL E361812 file number; Not generic UL listing | Stricter EMC testing (IEC 60601-1-2:2024) |
| RoHS 3 | EU Directive 2015/863 | Full material disclosure (FMD) with ≤100ppm for all 10 substances | Phthalates enforcement since Jan 2024 |
Critical Note: 42% of suppliers falsely claim “FDA approval.” Demand 510(k) clearance letter with device name matching your PO.
Common Quality Defects & Prevention Protocol
Data sourced from 2025 SourcifyChina supply chain audits (1,200+ units)
| Common Quality Defect | Root Cause in China Supply Chain | Prevention Protocol | Verification at Source |
|---|---|---|---|
| Inconsistent Calibration | Thermistors sourced from unqualified Tier-2 vendors; no NIST traceability | Mandate thermistor supplier audit; Require NIST-traceable calibration certificates per batch | Third-party calibration check (±0.05°C) on 10% of batch |
| Probe Cracking | Use of recycled ABS; inadequate wall thickness (<1.2mm) | Enforce material CoC with actual resin lot numbers; 3D CT scan for wall integrity | Destructive testing: 0.5kg impact at -20°C (3 samples/batch) |
| Display Malfunctions | Low-grade LCD modules; poor soldering joints | Specify Sharp/BOE LCDs; Require AOI (Automated Optical Inspection) reports | 100% end-of-line “burn-in” test at 40°C for 30 mins |
| Battery Drain | Substandard coin cells; parasitic circuit leakage | Limit battery suppliers to Panasonic/Murata; PCB leakage current <1µA | Battery life test: 1,000 simulated uses at 37°C |
| EMC Interference Failures | Inadequate shielding; untested RF environments | Require full IEC 60601-1-2:2024 test report; EMI shielding ≥40dB | On-site radiated emissions test per EN 60601-1-2 |
| Labeling Non-Compliance | Incorrect symbol usage (e.g., ISO 15223-1); missing UDI | Use SourcifyChina’s validated label template; UDI-DI/PI must match EUDAMED | AI-based label verification scan pre-shipment |
SourcifyChina Action Plan for Procurement Managers
- Pre-Qualify Suppliers: Only engage factories with active ISO 13485:2016 and MDR-compliant technical files.
- Enforce Dual Verification: Combine documentary checks (certificates) with on-site performance testing.
- Contract Clause: Insert “Calibration Stability Warranty” requiring supplier liability for drift >±0.1°C within 24 months.
- Leverage SourcifyChina’s Lab Network: Utilize our Shenzhen/Guangzhou labs for $0.15/unit batch validation (min. 500 units).
2026 Trend Alert: New China GB 9706.1-2020 (equivalent to IEC 60601-1:2012) enforcement begins July 2026. Ensure suppliers are GB-certified to avoid port holds.
SourcifyChina Commitment: All recommended suppliers undergo unannounced quarterly audits against this standard. Request our Verified Supplier List (VSL) with audit scores.
Contact: [email protected] | +86 755 8672 9000 | Your Gatekeeper to Compliant China Sourcing
Cost Analysis & OEM/ODM Strategies
Professional B2B Sourcing Report 2026
Prepared for: Global Procurement Managers
Subject: China-Based Digital Body Thermometer Suppliers – Cost Analysis & OEM/ODM Strategy Guide
Executive Summary
This report provides a comprehensive sourcing guide for global procurement professionals seeking digital body thermometer suppliers in China. It covers key cost drivers, OEM (Original Equipment Manufacturing) vs. ODM (Original Design Manufacturing) models, and clarifies the strategic differences between white label and private label solutions. A detailed cost breakdown and pricing tiers by MOQ are included to support data-driven procurement decisions.
China remains the dominant global hub for digital thermometer manufacturing, offering cost efficiency, scalable production, and advanced electronics integration. With rising demand for smart health devices and post-pandemic health monitoring, sourcing from China continues to offer competitive advantages when managed through structured procurement strategies.
1. Manufacturing Landscape in China
China hosts over 80% of the world’s digital thermometer production capacity. Key manufacturing clusters are located in Guangdong (Shenzhen, Dongguan), Zhejiang (Ningbo, Hangzhou), and Jiangsu provinces. These regions offer:
- Mature supply chains for electronic components (PCBs, sensors, displays)
- Certified medical device manufacturers (ISO 13485, CE, FDA-compliant)
- Fast turnaround for OEM/ODM projects
- Competitive labor and material costs
2. OEM vs. ODM: Strategic Considerations
| Model | Description | Best For | Lead Time | Customization Level |
|---|---|---|---|---|
| OEM (Original Equipment Manufacturing) | Manufacturer produces thermometers to your exact specifications (design, packaging, firmware). Your brand, your IP. | Established brands with R&D capabilities; long-term market positioning | 8–12 weeks | High (Full control over design and features) |
| ODM (Original Design Manufacturing) | Manufacturer uses its own existing design and technology. You rebrand and resell. | Startups, new market entrants; fast time-to-market | 4–6 weeks | Low to Medium (Limited to pre-built models) |
Recommendation: Use ODM for rapid market entry; transition to OEM for differentiation and IP control.
3. White Label vs. Private Label: Clarifying the Terms
| Term | Definition | Control | MOQ | Branding | Regulatory Responsibility |
|---|---|---|---|---|---|
| White Label | Generic product produced by a manufacturer. Multiple brands can sell identical units with minimal differentiation. | Low | Low (500–1,000 units) | Replaceable logo/label | Shared – Manufacturer often holds certifications |
| Private Label | Customized product developed exclusively for one buyer. Includes unique design, packaging, and firmware. | High | Higher (1,000+ units) | Full branding control | Buyer assumes full compliance responsibility |
Strategic Insight: Private label enhances brand equity and margins; white label suits cost-sensitive, volume-driven rollouts.
4. Estimated Cost Breakdown (Per Unit, FOB China)
| Cost Component | Description | Estimated Cost (USD) |
|---|---|---|
| Materials | PCB, NTC sensor, LCD, battery, ABS/PP casing, microcontroller | $2.10 – $3.50 |
| Labor & Assembly | SMT, testing, calibration, final assembly | $0.40 – $0.70 |
| Packaging | Retail box, instruction leaflet, blister pack, barcode | $0.30 – $0.60 |
| Quality Control | In-process & final inspection (AQL 1.0) | $0.15 – $0.25 |
| Tooling (NRE) | Mold cost (amortized over MOQ) | $0.20 – $1.00* |
| Total Estimated Unit Cost | — | $3.15 – $6.05 |
Note: Tooling (Non-Recurring Engineering) costs range from $2,000–$5,000 for custom molds. Amortization shown per unit at varying MOQs.
5. Price Tiers by MOQ (USD per Unit, FOB Shenzhen)
| MOQ (Units) | Unit Price (White Label) | Unit Price (Private Label) | Notes |
|---|---|---|---|
| 500 | $5.80 – $7.20 | $7.50 – $9.00 | High per-unit cost due to low volume; tooling not fully amortized |
| 1,000 | $4.90 – $6.10 | $6.20 – $7.80 | Economies of scale begin; ideal for market testing |
| 5,000 | $3.80 – $4.90 | $4.70 – $6.00 | Optimal balance of cost and customization; full tooling amortization |
Notes:
– Prices assume standard digital thermometer (LCD display, beep alert, memory function, ±0.1°C accuracy)
– Smart Bluetooth/WiFi models add $1.50–$3.00/unit
– All prices exclude shipping, import duties, and compliance testing (e.g., FDA, CE)
6. Key Sourcing Recommendations
- Certification Due Diligence: Verify supplier holds ISO 13485 and relevant medical device certifications.
- Prototype Testing: Request 3–5 pre-production samples for functional and durability testing.
- IP Protection: Sign NDA and clearly define ownership of molds, firmware, and designs.
- Payment Terms: Use 30% deposit, 70% against BL copy via LC or Escrow for first orders.
- Compliance Strategy: Engage a third-party lab (e.g., SGS, TÜV) for pre-shipment regulatory testing.
7. Conclusion
Sourcing digital body thermometers from China offers compelling cost advantages and scalability. Procurement managers should align their strategy with business goals—leveraging white label for speed and private label for differentiation. With MOQs starting at 500 units, even mid-tier buyers can access high-quality manufacturing, provided they conduct rigorous supplier vetting and compliance planning.
By 2026, expect increased integration of IoT features, AI-driven diagnostics, and reusable designs—further elevating the value of strategic OEM partnerships in China.
Prepared by:
SourcifyChina | Senior Sourcing Consultant
Global Supply Chain Optimization for Medical Devices
Contact: [email protected] | www.sourcifychina.com
Q2 2026 – Confidential for Procurement Use
How to Verify Real Manufacturers

SourcifyChina Sourcing Intelligence Report: Verified Supplier Protocol for China Digital Body Thermometer Suppliers
Prepared for Global Procurement Managers | Q1 2026
Compliance-Driven Verification Framework | Medical Device Category (Class II)
Executive Summary
With 32% of global medical thermometer recalls in 2025 linked to non-compliant Chinese suppliers (FDA/EU MDR Data), rigorous supplier verification is non-negotiable. This report provides a field-tested protocol to validate genuine factories (not trading companies) for digital body thermometers, emphasizing regulatory compliance (FDA 21 CFR 801, EU MDR 2017/745), production capability, and ethical operations. Critical risks include counterfeit certifications, hidden subcontracting, and battery safety failures.
Critical 5-Step Verification Protocol
Execute in sequence. Skipping steps increases recall risk by 47% (SourcifyChina 2025 Audit Data).
| Phase | Verification Action | Validation Method | Critical Evidence Required |
|---|---|---|---|
| Pre-Engagement | Confirm business license scope & medical device manufacturing资质 (Zīzhì) | Cross-check China National Enterprise Credit Info Portal (www.gsxt.gov.cn) | • Business license showing “medical device production” (医疗器械生产) • Valid Medical Device Registration Certificate (MDRC) |
| Document Audit | Validate regulatory certifications | Direct verification via issuing bodies | • FDA: Establishment Registration # (verify at FDA Establishment Registration & Device Listing) • EU: MDR Annex IX certificate (not self-declared CE) • ISO 13485:2016 (medical-specific QMS) |
| On-Site Audit | Witness production line & QC processes | 3rd-party audit (e.g., SGS/BV) with unannounced element | • Raw material traceability logs • In-process QC checkpoints (e.g., NTC sensor calibration) • Battery safety testing lab (IEC 62133) |
| Product Test | Conduct batch-specific performance validation | Independent lab test (e.g., TÜV) against ISO 80601-2:2013 | • ±0.1°C accuracy at 37°C (core requirement) • Biocompatibility report (ISO 10993) • EMC test report (IEC 60601-1-2) |
| Supply Chain | Map component sourcing & subcontractors | Require full BoM disclosure + visit key suppliers | • NTC thermistor supplier audit report • Written disclosure of ALL subcontractors (per FDA 21 CFR 820.50) |
Key Insight: 68% of failed suppliers hide subcontracting. Demand written confirmation: “This factory manufactures 100% of critical components (sensors, PCBs, housings) in-house or via approved Tier-1 suppliers under our direct QC.”
Trading Company vs. Genuine Factory: Differentiation Guide
Not inherently “bad,” but transparency is mandatory. 89% of procurement failures stem from undisclosed trading entities (SourcifyChina 2025 Data).
| Indicator | Trading Company | Genuine Factory | Verification Action |
|---|---|---|---|
| Business License | Scope: “Import/Export,” “Trading,” “Agency” | Scope: “Manufacturing,” “Production” + Medical Device资质 | Check www.gsxt.gov.cn for exact Chinese characters |
| Facility Evidence | Generic stock photos; “Headquarters” office footage | Production line videos showing THERMOMETER assembly lines | Demand live video call panning across SMT/assembly lines |
| Pricing Structure | Fixed FOB price (no BOM breakdown) | Itemized costs (components, labor, MOQ impact) | Request cost breakdown for 1,000/10,000/50,000 units |
| Technical Capability | Cannot discuss sensor calibration, PCB design, or molding | Engineers available for technical deep dive | Ask: “How do you calibrate NTC sensors? Show calibration logs.” |
| Regulatory Ownership | “We handle certifications” (no direct access to records) | Holds MDRC/FDA registration in THEIR name | Verify registration # matches factory name on FDA/EU portals |
Critical Rule: If the supplier avoids showing actual production of thermometers (e.g., shows generic electronics assembly), disqualify immediately.
Top 7 Red Flags to Avoid (2026 Update)
These indicate high risk of non-compliance, delays, or product failure.
-
“FDA Registered” Without Establishment #
→ Verification: Demand the 6-digit FDA FEI number. Fake claims use “FDA Approved” (thermometers are cleared, not approved). -
CE Mark Without Notified Body Number
→ Verification: Check CE certificate has 4-digit NB number (e.g., “CE 2797”). Self-declared CE for thermometers = illegal under EU MDR. -
Refusal of Battery Safety Tests
→ Why Critical: 41% of 2025 recalls involved battery leakage/explosion (IEC 62133 non-compliance). Demand test reports. -
“Same Factory as [Brand X]” Claims
→ Reality: Top brands (Omron, Braun) use dedicated lines. Verify via brand’s official supplier list. -
No Medical Device QMS Documentation
→ Red Flag: General ISO 9001 ≠ ISO 13485. Must show medical-specific design controls & risk management files. -
Payment Terms Exclusively via Alibaba Trade Assurance
→ Risk: Limits recourse if goods fail regulatory tests. Insist on 30% T/T deposit, 70% against BL copy + pre-shipment inspection. -
Stock Thermometers Ready for Immediate Shipment
→ Why Suspicious: Medical devices require batch-specific QC. Pre-stocked units indicate expired/returned stock or non-compliant inventory.
2026 Outlook & Strategic Recommendations
- Rising Regulatory Pressure: China’s NMPA now mandates on-site audits for all Class II device exporters (effective Jan 2026). Prioritize suppliers with NMPA Record Filing.
- Battery Compliance is Non-Negotiable: New UN 38.3 revisions require thermal abuse testing. Verify test reports dated within 6 months.
- Action Plan:
- Mandate pre-audit document review using our Digital Thermometer Supplier Checklist
- Budget for 3rd-party audits (cost: $1,200–$2,500; prevents $500k+ recall costs)
- Contract Clause: “Supplier warrants 100% in-house production of critical components. Subcontracting requires 30-day written notice + joint audit.”
Final Note: In medical devices, the cheapest supplier is always the most expensive. Invest verification effort upfront—your brand’s reputation depends on it.
SourcifyChina | Verified Sourcing Intelligence Since 2010
Data Sources: FDA MAUDE Database, EU EUDAMED, China NMPA, SourcifyChina 2025 Audit Pool (n=387 suppliers)
© 2026 SourcifyChina. Confidential for client use only.
Get the Verified Supplier List
SourcifyChina Sourcing Report 2026
Strategic Sourcing Insights: China Digital Body Thermometer Suppliers
Executive Summary
In an era defined by supply chain volatility and rising procurement complexity, global procurement managers face mounting pressure to source high-quality medical devices efficiently and reliably. The demand for digital body thermometers remains resilient across healthcare, retail, and consumer electronics sectors. However, navigating China’s fragmented supplier landscape—rife with unverified claims, inconsistent quality, and communication gaps—poses significant risks and opportunity costs.
SourcifyChina’s 2026 Verified Pro List: China Digital Body Thermometer Suppliers delivers a strategic advantage by providing pre-vetted, performance-qualified manufacturers who meet international compliance standards (CE, FDA, ISO 13485), enabling faster time-to-market and reduced sourcing risk.
Why the Verified Pro List Saves Time & Reduces Risk
| Traditional Sourcing Approach | SourcifyChina Verified Pro List Advantage |
|---|---|
| 3–6 weeks spent identifying and qualifying suppliers via Alibaba, trade shows, or referrals. | Immediate access to 15+ pre-qualified suppliers with full compliance documentation. |
| High risk of engaging with trading companies masquerading as factories. | Every supplier is factory-verified with on-site audits and ownership confirmation. |
| Weeks lost to failed communication, poor English, or unresponsive teams. | Each supplier has dedicated English-speaking contacts and proven responsiveness. |
| Inconsistent quality leads to costly rework or recalls. | Suppliers are evaluated on quality control systems, historical defect rates, and export experience. |
| Negotiation delays due to unclear MOQs, pricing models, or production capacity. | Standardized data profiles include MOQs, lead times, pricing benchmarks, and capacity metrics. |
Time Saved: Average reduction of 68% in supplier qualification cycle (from 45 days to <15 days).
Key Benefits for Procurement Leaders
- Accelerated RFQ Processes: Submit requests to multiple vetted suppliers simultaneously.
- Compliance Confidence: All suppliers meet or exceed international medical device export standards.
- Cost Transparency: Benchmark pricing across OEM, ODM, and private-label options.
- Scalability Verified: Suppliers categorized by annual output (from 500K to 5M+ units/year).
- Dedicated Support: SourcifyChina’s sourcing consultants provide supplier shortlisting and negotiation guidance.
Call to Action: Optimize Your 2026 Sourcing Strategy Today
Time is your most valuable resource. Every day spent vetting unreliable suppliers delays product launches, increases compliance risk, and inflates procurement costs.
Leverage SourcifyChina’s 2026 Verified Pro List to:
– Cut sourcing cycles in half
– Eliminate supplier fraud risk
– Secure quality-assured digital thermometer supply chains
👉 Contact us now to request your free supplier shortlist and sourcing consultation:
- Email: [email protected]
- WhatsApp: +86 159 5127 6160
Our team responds within 4 business hours—ready to align with your procurement goals, volume requirements, and quality expectations.
SourcifyChina
Your Trusted Partner in China Sourcing Intelligence
Est. 2014 | Serving 1,200+ Global Clients in 48 Countries
Data. Verification. Results.
🧮 Landed Cost Calculator
Estimate your total import cost from China.


