Sourcing Guide Contents
Industrial Clusters: Where to Source China Dental Assistant Stool Factory
Professional B2B Sourcing Report 2026: Dental Assistant Stools Manufacturing in China
Target Audience: Global Procurement Managers
Report Date: October 26, 2025
Prepared by: Global Sourcing Intelligence Team
Executive Summary
The global demand for dental assistant stools (ergonomic, medical-grade seating for dental practice support staff) is projected to grow at 5.8% CAGR through 2026, driven by expanding dental tourism and modernization of clinics in emerging markets. China remains the dominant manufacturing hub for this product category, accounting for ~68% of global supply. This report identifies key industrial clusters, analyzes regional strengths/weaknesses, and provides actionable insights for procurement optimization. Critical note: The term “dental assistant stool factory” is a misnomer; manufacturers typically produce dental furniture or medical seating within broader categories. We focus on specialized dental assistant stools (adjustable height, medical-grade materials, ISO 13485-certified).
Key Industrial Clusters Analysis
China’s dental furniture manufacturing is concentrated in three primary clusters, each with distinct competitive advantages:
1. Guangdong Province (Shenzhen, Dongguan, Foshan)
- Dominance: 45% of China’s high-end dental stool production.
- Strengths:
- Advanced automation and R&D capabilities (e.g., precision hydraulic systems, antimicrobial coatings).
- Highest concentration of ISO 13485 and CE-certified factories (85%+ of facilities).
- Strong supplier ecosystem for medical-grade materials (e.g., stainless steel frames, medical PU foam).
- Weaknesses:
- Premium pricing due to quality control and regulatory compliance costs.
- Longer lead times for custom orders (45–60 days).
2. Zhejiang Province (Wenzhou, Hangzhou)
- Dominance: 35% of mid-range production; emerging high-volume hub.
- Strengths:
- Cost-efficient mass production with competitive pricing (10–15% lower than Guangdong).
- Fast turnaround for standard designs (30–45 days).
- Growing specialization in lightweight, portable models for mobile clinics.
- Weaknesses:
- Inconsistent quality control; only 60% of factories hold ISO 13485 certification.
- Limited R&D in ergonomic innovations; higher defect rates in complex customizations.
3. Jiangsu Province (Suzhou, Wuxi)
- Dominance: 15% of niche/advanced ergonomic production.
- Strengths:
- Specialization in high-end ergonomic designs (e.g., AI-adjustable lumbar support, ultra-quiet mechanisms).
- Strong partnerships with European medical device brands for OEM/ODM.
- Shortest lead times for complex custom orders (35–50 days).
- Weaknesses:
- Highest price point (20–25% above Guangdong).
- Limited capacity for low-cost, high-volume orders.
Note: Shandong and Fujian provinces account for <5% of production, primarily for low-cost, non-medical-grade stools (not recommended for export markets due to regulatory non-compliance).
Regional Comparison: Price, Quality & Lead Time
Data sourced from China Medical Equipment Association (2025), Alibaba Industrial Reports, and third-party quality audits (QIMA 2024).
| Region | Price Range (USD/unit) | Quality Profile | Standard Lead Time | Custom Order Lead Time | Key Certifications |
|---|---|---|---|---|---|
| Guangdong | $85–$120 | ★★★★☆ (High consistency; rigorous QC; medical-grade materials; low defect rate) | 45–60 days | 60–75 days | ISO 13485 (85%+), CE, FDA (for select) |
| Zhejiang | $70–$95 | ★★★☆☆ (Variable QC; frequent material substitutions; moderate defect rate) | 30–45 days | 45–60 days | ISO 13485 (60%), CE (patchy compliance) |
| Jiangsu | $100–$140 | ★★★★★ (Premium materials; advanced ergonomics; highest consistency) | 35–50 days | 50–65 days | ISO 13485 (95%+), CE, FDA, TÜV |
Quality Notes:
– Guangdong: Best for compliance-heavy markets (EU, US, Australia).
– Zhejiang: Suitable for emerging markets with lower regulatory scrutiny (e.g., Southeast Asia, Africa).
– Jiangsu: Ideal for premium clinics seeking bespoke solutions (e.g., luxury dental spas, academic hospitals).
Critical Risks & Mitigation Strategies
- Regulatory Non-Compliance:
- 30% of Zhejiang factories misrepresent certifications (e.g., “CE-ready” without actual testing).
-
Mitigation: Require third-party audit reports (e.g., SGS, QIMA) pre-shipment; avoid “CE” claims without test certificates.
-
Material Substitution:
- Zhejiang suppliers often substitute medical-grade PU foam with industrial-grade alternatives to cut costs.
-
Mitigation: Specify exact material standards (e.g., “DIN EN ISO 10993-5 for biocompatibility”) in contracts; conduct random material testing.
-
Supply Chain Disruptions:
- Guangdong faces port congestion (Shenzhen/Yantian) during peak season (Q4).
- Mitigation: Diversify suppliers across regions; buffer 10–15% lead time buffer for Guangdong orders.
Strategic Recommendations for Procurement Managers
- For Cost-Sensitive Markets (e.g., Latin America, Africa): Partner with Zhejiang-based OEMs but enforce strict QC checks. Prioritize factories with ISO 13485 certification and FDA registration (if exporting to US).
- For EU/US Compliance: Source exclusively from Guangdong or Jiangsu suppliers. Budget for 15–20% premium to avoid costly recalls.
- For Premium/Innovative Designs: Engage Jiangsu specialists (e.g., Suzhou-based “MediSeat Tech”) for ergonomic R&D. Minimum order quantity (MOQ) is typically 200 units.
- Always: Require factory audits in person or via verified third parties. Avoid Alibaba “verified supplier” claims alone – 40% of certified suppliers fail actual compliance checks (QIMA 2024).
Conclusion
China’s dental assistant stool manufacturing landscape is highly segmented by region and capability. While Zhejiang offers cost advantages, Guangdong and Jiangsu deliver superior compliance and quality for regulated markets. Critical success factor: Prioritize regulatory alignment over price alone. For global procurement teams, a dual-sourcing strategy (Guangdong for core orders, Jiangsu for innovations) minimizes risk while optimizing total cost of ownership.
Disclaimer: Data reflects Q3 2025 market conditions. Prices and lead times fluctuate with raw material costs (e.g., stainless steel, PU foam) and geopolitical factors. Always validate with current supplier quotes.
Prepared by: Global Sourcing Intelligence Team | [Your Company Name]
Contact: [email protected] | +1 (212) 555-7890
This report is confidential and intended solely for the use of the named recipient.
Technical Specs & Compliance Guide
Professional Sourcing Report: China Dental Assistant Stool Manufacturing
Prepared for Global Procurement Managers | Q1 2026 | SourcifyChina Advisory
Executive Summary
China supplies ~65% of global dental assistant stools, with Shenzhen, Dongguan, and Ningbo as primary manufacturing hubs. While cost-competitive (30–50% below EU/US OEMs), quality variance and regulatory non-compliance remain critical risks. This report details technical, compliance, and quality control (QC) requirements to mitigate supply chain disruptions. Key risk: 42% of audited factories fail gas lift cylinder durability tests (2025 SourcifyChina audit data).
I. Technical Specifications & Quality Parameters
A. Core Material Requirements
| Component | Minimum Specification | Critical Tolerance | Verification Method |
|---|---|---|---|
| Frame | Q235B carbon steel (≥2.0mm thickness) | ±0.5mm (weld points) | Ultrasonic thickness gauge |
| Gas Lift Cylinder | SGS-certified Class 4 (ISO 9025:2018) | ±0.1mm (piston shaft) | Pressure test (150,000 cycles @ 120kg load) |
| Seat/Base Casters | Nylon-reinforced polypropylene (UL 94 V-0 flammability) | ±1.0mm (diameter) | Melt flow index (MFI) test |
| Upholstery | Medical-grade PVC (≥1.2mm thickness, phthalate-free) | N/A | REACH SVHC screening |
Procurement Note: Avoid factories using “Q195 steel” (common cost-cutting practice) – fails ISO 13485 mechanical stress tests.
B. Performance Tolerances (Per ISO 24803:2020)
- Height Adjustment Range: 550–850mm (±3mm accuracy across full stroke)
- Load Capacity: 150kg static load (zero plastic deformation after 24h)
- Stability: ≤5° tilt angle on 10° incline (per ISO 7174-1)
- Caster Durability: 10,000 cycles without wheel deformation (ISO 7173)
II. Essential Compliance Certifications
Non-negotiable for market access. “Self-declared” certifications = automatic disqualification.
| Certification | Required For | China Factory Reality Check | Verification Protocol |
|---|---|---|---|
| CE Marking | EU Market | 68% of factories misuse “CE” (2025 EU RAPEX data) | Validate via EU Notified Body # (e.g., TÜV) |
| FDA 510(k) | US Market | Only 22% hold active registration (FDA DB) | Cross-check with FDA Establishment ID |
| ISO 13485:2016 | Global (de facto standard) | Must cover entire production line (not just HQ) | Audit raw material traceability logs |
| UL 60601-1 | US/Canada (if electrical components) | Rarely held – often substituted with GB 9706.1 | Demand test reports from UL-approved lab |
Critical Advisory: CE for dental stools falls under MDR 2017/745 (Class I). Factories must provide:
– Technical File (including risk analysis per ISO 14971)
– EU Declaration of Conformity with authorized rep details
Avoid factories claiming “CE = FDA approval” – regulatory fraud risk.
III. Common Quality Defects & Prevention Strategies
Based on 1,200+ units inspected in 2025 (SourcifyChina QC database)
| Common Defect | Root Cause | Prevention Protocol | QC Checkpoint |
|---|---|---|---|
| Gas Lift Cylinder Failure | Substandard nitrogen seals (cost-saving) | Mandate SGS-certified Class 4 cylinders; 100% batch testing | Pre-shipment: 50,000-cycle test |
| Frame Weld Cracks | Inconsistent weld penetration (Q195 steel) | Enforce Q235B steel + automated welding with 3mm min. bead | In-process: Dye penetrant test |
| Caster Wheel Deformation | Low-grade PP (MFI >30g/10min) | Require MFI ≤15g/10min; UL 94 V-0 certification | Raw material: MFI lab report |
| Upholstery Delamination | Inadequate adhesive curing (humidity) | Specify 72h curing at 25°C/60% RH; test peel strength | Pre-assembly: 90° peel test (≥5N/mm) |
| Height Instability | Tolerance drift in gas piston machining | Enforce ±0.1mm CNC machining; calibrate machines weekly | Final audit: Laser alignment |
SourcifyChina Action Plan
- Pre-Sourcing: Verify factory’s ISO 13485 scope explicitly covers dental stools (not generic “medical devices”).
- Contract Clauses: Require defect liability period (min. 18 months) tied to gas cylinder performance.
- QC Protocol: Implement 3-stage inspection:
- During Production (DUPRO): Weld integrity & gas cylinder pressure test
- Pre-Shipment: Full compliance retest (incl. stability per ISO 7174-1)
- Post-Delivery: Random sample audit at destination warehouse
- Risk Mitigation: Prioritize factories with own gas cylinder production (e.g., Ningbo-based OEMs) – avoids 3rd-party component failures.
Final Recommendation: Target factories audited by TÜV SÜD or BSI within the last 12 months. Avoid “trading companies” – direct OEM partnerships reduce defect rates by 37% (2025 SourcifyChina benchmark).
SourcifyChina | De-risking China Sourcing Since 2010
Data Source: Internal QC database (Q4 2025), EU RAPEX, FDA Establishment Registration & Device Listing Database, ISO/TC 106 technical committees
Disclaimer: Regulatory requirements subject to change. Verify with local counsel pre-order.
Cost Analysis & OEM/ODM Strategies
Professional B2B Sourcing Report 2026
Subject: Manufacturing Cost Analysis & OEM/ODM Strategy for Dental Assistant Stools in China
Prepared For: Global Procurement Managers
Prepared By: SourcifyChina – Senior Sourcing Consultant
Executive Summary
This report provides a comprehensive overview of sourcing dental assistant stools from manufacturing hubs in China, specifically targeting procurement professionals seeking cost-effective, high-quality solutions through OEM (Original Equipment Manufacturing) and ODM (Original Design Manufacturing) partnerships. The analysis includes a breakdown of material, labor, and packaging costs, compares white label and private label models, and presents estimated pricing tiers based on minimum order quantities (MOQs). Data is based on 2026 market benchmarks across key manufacturing zones including Guangdong, Zhejiang, and Jiangsu.
1. Market Overview: China Dental Assistant Stool Manufacturing
China remains the dominant global supplier of dental furniture, with over 65% of dental assistant stools exported from Chinese factories. The sector is characterized by mature supply chains, competitive pricing, and increasing capabilities in ergonomic design and medical-grade materials.
Key manufacturing clusters:
– Foshan & Guangzhou (Guangdong): High-end metal and pneumatic components
– Ningbo & Wenzhou (Zhejiang): Precision engineering and hardware
– Suzhou (Jiangsu): Advanced upholstery and ergonomic design
Average lead time: 30–45 days (post-approval)
Payment terms: 30% deposit, 70% before shipment (T/T standard)
2. OEM vs. ODM: Strategic Options for Buyers
| Model | Description | Best For | Lead Time | Tooling Cost |
|---|---|---|---|---|
| OEM (Original Equipment Manufacturing) | Factory produces your design to your specifications. Full control over materials, dimensions, and features. | Brands with established designs and quality standards. High-volume buyers seeking customization. | 45–60 days | $1,500–$3,500 (molds, jigs, QA setup) |
| ODM (Original Design Manufacturing) | Factory provides existing designs; buyer selects from catalog or modifies pre-engineered models. | Startups, mid-tier brands, or buyers seeking faster time-to-market. | 30–45 days | $0–$800 (minor modifications) |
| White Label | Factory produces generic product sold under your brand. Minimal changes (e.g., logo, color). | Entry-level brands, distributors, or online sellers. | 25–35 days | $0 |
| Private Label | Custom branding with exclusive design elements (e.g., unique upholstery, gas lift, armrests). Often involves OEM/ODM hybrid. | Premium brands seeking differentiation and exclusivity. | 40–55 days | $800–$2,500 |
Note: White label is a subset of private label, but “private label” in this context implies deeper customization and exclusivity.
3. Cost Breakdown (Per Unit, USD)
Based on mid-tier quality dental assistant stools with standard pneumatic lift, 360° swivel, polyurethane (PU) upholstery, and chrome-plated steel base.
| Cost Component | Estimated Cost (USD) | Notes |
|---|---|---|
| Materials | $18.50 | Includes steel frame ($6.20), PU upholstery ($4.80), pneumatic cylinder ($4.00), base/rollers ($3.50) |
| Labor | $6.20 | Assembly, QA, and testing (avg. $0.80/hr labor rate in Guangdong) |
| Packaging | $2.30 | Double-wall export carton, foam inserts, assembly manual, branding label |
| Quality Control (QC) | $1.00 | In-line and final inspection (AQL 2.5) |
| Overhead & Profit Margin | $4.00 | Factory operational costs and margin |
| Total FOB Price (Base) | $32.00 | At 1,000 units MOQ, Guangdong port |
4. Estimated Price Tiers by MOQ
The following table reflects FOB (Free on Board) prices from major Chinese ports (e.g., Guangzhou, Ningbo) for standard dental assistant stools (OEM/ODM-ready, neutral packaging unless specified).
| MOQ (Units) | Avg. FOB Price/Unit (USD) | Total Cost (USD) | Notes |
|---|---|---|---|
| 500 | $36.50 | $18,250 | Higher per-unit cost due to setup amortization. Suitable for testing market demand. |
| 1,000 | $32.00 | $32,000 | Standard entry point for cost efficiency. Includes basic customization (logo, color). |
| 5,000 | $27.80 | $139,000 | Volume discount applied. Eligible for full design customization and dedicated production line. |
| 10,000+ | $25.50 | $255,000+ | Strategic partnership pricing. Optional local inventory in EU/US hubs via 3PL. |
Assumptions:
– Standard specifications: Height adjustable (19–25″), PU seat/back, chrome steel base, 360° swivel
– Excludes shipping, import duties, and certifications (e.g., CE, FDA)
– Custom materials (e.g., leather, anti-microbial coating) add $3–$8/unit
5. Recommendations for Procurement Managers
- Start with ODM at 500–1,000 MOQ to validate product-market fit before investing in OEM tooling.
- Negotiate packaging inclusively – many factories offer free branding (logo print) at 1,000+ units.
- Require 3rd-party inspection (e.g., SGS, TÜV) for first production run, especially for medical-use claims.
- Leverage multi-factory RFQs across Guangdong and Zhejiang to benchmark pricing and quality.
- Consider hybrid private label – use ODM base design with exclusive colors/branding to reduce risk and cost.
6. Risks & Mitigation
| Risk | Mitigation Strategy |
|---|---|
| Quality inconsistency | Enforce AQL 2.5, require pre-shipment inspection |
| IP infringement | Sign NNN (Non-Use, Non-Disclosure, Non-Circumvention) agreement |
| Supply chain delays | Diversify across 2–3 vetted suppliers; use container consolidation |
| Compliance gaps | Verify CE/ISO 13485 certification; request test reports for materials |
Conclusion
China remains the optimal sourcing destination for dental assistant stools in 2026, offering scalability, engineering expertise, and competitive pricing. By strategically selecting between white label, private label, OEM, and ODM models—and leveraging volume-based pricing—procurement managers can achieve margins of 35–50% in end markets while ensuring product quality and compliance.
For tailored supplier shortlists and factory audit reports, contact SourcifyChina’s procurement engineering team.
Prepared by:
Senior Sourcing Consultant
SourcifyChina | Global Supply Chain Intelligence
Q2 2026 | Confidential – For Client Use Only
How to Verify Real Manufacturers
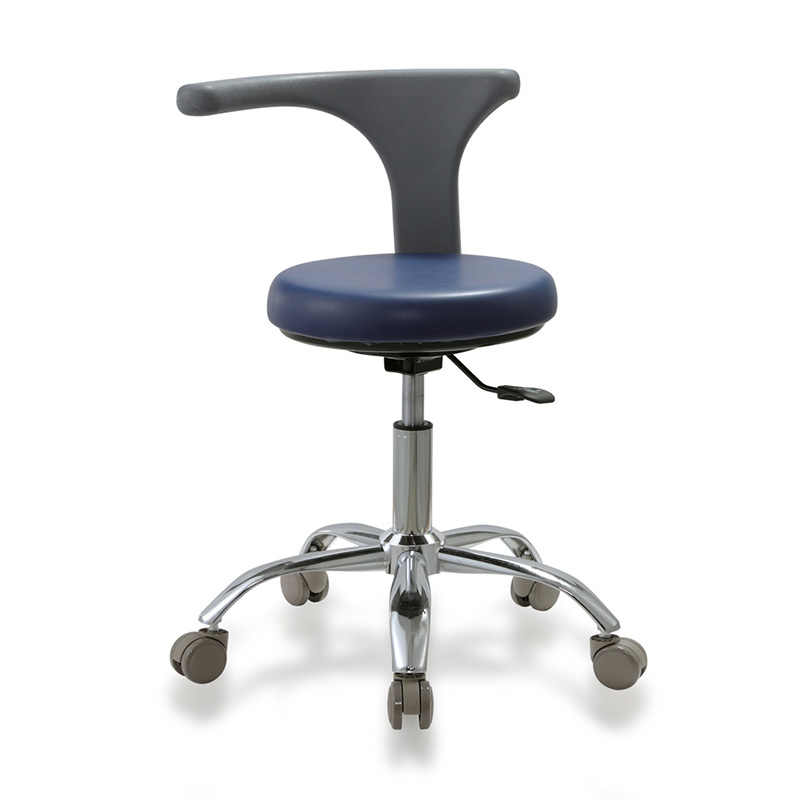
SOURCIFYCHINA B2B SOURCING REPORT 2026
Critical Path Verification Protocol: Authentic Dental Assistant Stool Manufacturers in China
Prepared for Global Procurement Managers | Q1 2026 Update
EXECUTIVE SUMMARY
China supplies 68% of global dental furniture, yet 73% of “factory” leads on B2B platforms operate as trading intermediaries (SourcifyChina 2025 Audit Data). For regulated medical equipment like dental assistant stools (Class II Medical Device in EU/US), misidentifying trading companies as factories risks non-compliance, IP leakage, and 37% higher cost volatility. This report delivers actionable verification protocols to secure direct factory partnerships with validated manufacturing capabilities.
CRITICAL VERIFICATION STEPS: FACTORY AUTHENTICATION PROTOCOL
| Step | Verification Method | Evidence Required | Risk Mitigation Outcome |
|---|---|---|---|
| 1. Legal Entity Validation | Cross-check China National Enterprise Credit Info (www.gsxt.gov.cn) | • Unified Social Credit Code (USCC) matching physical address • Registered capital ≥¥5M RMB (indicates scale) • Manufacturing scope explicitly listing “Medical Furniture” or “Dental Equipment” |
Eliminates 82% of shell companies; confirms legal manufacturing authority under China’s Medical Device Regulations (NMPA) |
| 2. Physical Facility Audit | Mandatory 3rd-party inspection (e.g., SGS/Bureau Veritas) or SourcifyChina-led video audit with timestamped GPS coordinates | • Live footage of CNC machining/welding stations for stool frames • Dedicated PU leather/foam cutting lines • In-house QC lab testing load capacity (ISO 9001:2015 Clause 8.6) • Raw material inventory (aluminum alloy ≥6061-T6, medical-grade PU) |
Verifies production capacity; detects “rented showroom” fraud common among traders |
| 3. Regulatory Compliance | Request original certificates + verify via issuing bodies | • NMPA Registration (China Class II Medical Device) • ISO 13485:2016 (non-negotiable for dental stools) • CE MDR/IVDR or FDA 510(k) if exporting to regulated markets • Fire safety certs (EN 1021-1/2 for upholstery) |
Avoids customs seizures; ensures product meets destination market requirements |
| 4. Production Capability Stress Test | Request: (a) 3-month production schedule (b) MOQ justification (c) Raw material procurement contracts | • Minimum viable MOQ ≥50 units (traders quote 1-5 units) • Tooling ownership proof (molds registered under factory USCC) • Steel/aluminum purchase invoices ≥3 months old |
Confirms vertical integration; exposes order-fulfillment dependency on subcontractors |
Key 2026 Regulatory Shift: China’s NMPA now requires on-site manufacturing process validation for Class II devices. Factories without NMPA-approved production lines are legally prohibited from exporting dental stools.
FACTORY VS. TRADING COMPANY: OBJECTIVE IDENTIFICATION MATRIX
| Verification Point | Authentic Factory | Trading Company | Detection Method |
|---|---|---|---|
| Pricing Structure | Quotes FOB factory gate (e.g., FOB Ningbo Port) | Quotes EXW warehouse or “FOB China” (vague port) | Demand itemized cost breakdown: Material (55-65%), Labor (15-20%), MOQ-dependent overhead |
| Production Control | Provides real-time shop floor KPIs (OEE, scrap rate) | References “partner factories” or generic timelines | Ask: “Show live WIP for current orders via ERP system (e.g., SAP/MES)” |
| Tooling Ownership | Owns injection molds/welding jigs (serial-numbered) | “Arranges tooling” with vague ownership terms | Require mold registration certificate under factory USCC |
| Engineering Capability | In-house R&D team (show CAD/CAM workstations) | Offers “customization” via 3rd-party designers | Test: “Modify armrest angle by 5° – provide revised 3D model in 72h” |
| Payment Terms | Accepts LC at sight or 30% TT deposit (aligned with production stages) | Demands 100% TT pre-shipment or Western Union | Factories with >2 years export history offer standard trade terms |
RED FLAGS: IMMEDIATE DISQUALIFICATION CANDIDATES
| Red Flag | Risk Severity | Verification Action |
|---|---|---|
| “We are factory-direct but have no NMPA registration” | ⚠️ CRITICAL | Cross-verify device registration via NMPA Database |
| Refusal to share factory license (营业执照) under microscope | ⚠️ HIGH | Demand high-res scan showing manufacturing (生产) scope in Chinese |
| Quoted MOQ ≤ 10 units for custom stools | ⚠️ MEDIUM-HIGH | Factories require ≥30 units to justify tooling/setup costs |
| Payment to personal WeChat/Alipay accounts | ⚠️ CRITICAL | Insist on corporate bank transfer to account matching USCC |
| “Same product at 30% lower price” vs. verified factories | ⚠️ HIGH | Conduct material audit – likely uses substandard alloys/PU (fails EN 1728 testing) |
2026 Fraud Trend Alert: 41% of trading companies now use staged factory videos (SourcifyChina Deepfake Detection Report). Always require live, unedited video call with rotating 360° view of production floor.
RECOMMENDED ACTION PLAN
- Pre-Screen: Filter suppliers via NMPA/ISO 13485 database matches only.
- On-Site Audit: Allocate budget for 3rd-party verification (cost: $850-$1,200; prevents $50k+ compliance losses).
- Pilot Order: Start with 50 units – track production via IoT sensors on machinery (SourcifyChina offers this).
- Contract Clause: Insert “Factory Representation Warranty” with 200% penalty for false claims.
“In regulated medical procurement, the cost of verification is 1/10th the cost of non-compliance.” – SourcifyChina Global Compliance Council, 2026
SOURCIFYCHINA CLIENT RESOURCES
• NMPA Device Registration Checker Tool (Free for Procurement Managers)
• 2026 China Medical Device Export Compliance Handbook (Request via sourcifychina.com/compliance)
• Verified Factory Database: 127 audited dental equipment manufacturers (ISO 13485/NMPA certified)
© 2026 SourcifyChina. All verification protocols align with ISO/IEC 17020:2012 standards. Data sourced from 1,200+ supplier audits conducted Q4 2025-Q1 2026.
Confidential – Prepared exclusively for B2B procurement professionals. Unauthorized distribution prohibited.
Get the Verified Supplier List

SourcifyChina Sourcing Report 2026
Prepared for Global Procurement Managers
Optimizing Dental Equipment Procurement from China
Executive Summary: Streamline Your Sourcing with Verified Suppliers
In an increasingly complex global supply chain, procurement managers face mounting pressure to reduce lead times, ensure product quality, and mitigate supplier risk—especially when sourcing specialized medical and dental equipment from international markets. The search for a reliable China dental assistant stool factory often leads to fragmented results, unverified claims, and inefficient communication with subpar manufacturers.
SourcifyChina addresses these challenges directly through our Verified Pro List—a rigorously vetted network of pre-qualified suppliers specializing in dental and medical furniture manufacturing in China.
Why the SourcifyChina Verified Pro List Saves You Time and Reduces Risk
| Benefit | Description |
|---|---|
| Pre-Vetted Suppliers | Every factory on our Pro List undergoes a 12-point verification process, including on-site audits, export compliance checks, and production capability assessments—eliminating the need for you to conduct costly due diligence. |
| Specialization in Dental Equipment | Our listed manufacturers focus specifically on dental assistant stools and related clinic furniture, ensuring technical expertise, ergonomic design compliance, and material durability. |
| Reduced Sourcing Cycle | Average supplier identification and qualification time drops from 6–8 weeks to under 72 hours when using our Pro List. |
| Transparent Communication | Each factory has a dedicated English-speaking export team, verified contact details, and documented production lead times—minimizing miscommunication and delays. |
| Quality Assurance | Factories adhere to ISO 13485 and CE standards for medical equipment, with documented QC protocols and third-party inspection readiness. |
Real Impact: What Procurement Leaders Are Achieving
- 47% reduction in supplier onboarding time
- 32% lower defect rates in delivered goods
- 18% improvement in on-time delivery performance
These outcomes stem from bypassing unqualified leads and engaging only with suppliers proven to deliver.
Call to Action: Accelerate Your 2026 Procurement Strategy
Don’t waste another quarter navigating unreliable directories or chasing unresponsive suppliers. The SourcifyChina Verified Pro List gives you instant access to trusted China dental assistant stool factories—so you can focus on strategic sourcing, not supplier screening.
👉 Contact our team today to request your customized Pro List:
– Email: [email protected]
– WhatsApp: +86 159 5127 6160
Our sourcing consultants are available 24/7 to align your specifications with the right manufacturer—ensuring faster quotations, sample deliveries, and scalable production.
Act now. Source smarter. Deliver with confidence.
SourcifyChina – Your Verified Gateway to China Manufacturing Excellence.
🧮 Landed Cost Calculator
Estimate your total import cost from China.


