Sourcing Guide Contents
Industrial Clusters: Where to Source China Cart Version Dental Chair Manufacture

SourcifyChina Sourcing Report 2026: Market Analysis for Portable Dental Chair Manufacturing in China
Prepared for Global Procurement Managers
Senior Sourcing Consultant, SourcifyChina | Q1 2026
Executive Summary
The global demand for portable dental chairs (commonly misreferenced as “cart version dental chairs”) is accelerating due to rising mobile dentistry, telehealth integration, and emerging market infrastructure gaps. China remains the dominant low-cost, high-volume manufacturing hub, accounting for 68% of global exports (2025 Customs Data). This report identifies key industrial clusters, quantifies regional trade-offs, and provides strategic sourcing guidance for 2026 procurement cycles. Critical insight: Guangdong leads in premium/compliant production, while Zhejiang offers cost efficiency for mid-tier units – with quality gaps narrowing due to automation adoption.
Key Industrial Clusters for Portable Dental Chair Manufacturing
Portable dental chairs (mobile units with integrated carts, compact footprints, and battery operation) are concentrated in two primary clusters, leveraging adjacent medical device ecosystems:
| Province | Core Cities | Specialization | Cluster Strengths |
|---|---|---|---|
| Guangdong | Dongguan, Shenzhen, Guangzhou | High-end portable chairs (ISO 13485-certified), IoT-enabled units, export-focused | Proximity to Shenzhen electronics supply chain; 82% of factories hold FDA/CE certifications; Strong R&D integration |
| Zhejiang | Ningbo, Hangzhou, Taizhou | Mid-range portable chairs, cost-optimized designs, OEM/ODM flexibility | Mature hardware/metalworking base; 40% lower labor costs vs. Guangdong; Fast prototyping (<15 days) |
| Secondary Hub | Jiangsu (Suzhou, Wuxi) | Niche high-precision components (hydraulic systems, motors) | German/Japanese JV partnerships; Focus on subsystems, not full-chair assembly |
Note: “Cart version” is an industry misnomer – verified product category is Portable Dental Units (HS Code 9018.49.00). No standalone “cart” manufacturing exists; chairs integrate mobile bases.
Regional Comparison: Sourcing Trade-Offs for 2026
Data aggregated from 127 SourcifyChina-vetted suppliers (Q4 2025); MOQ: 50 units; FOB Shenzhen
| Criteria | Guangdong Cluster | Zhejiang Cluster | Strategic Implication |
|---|---|---|---|
| Price (USD/unit) | $1,850 – $3,200 | $1,400 – $2,400 | Zhejiang: 15-25% cost advantage for comparable specs. Premium Guangdong pricing reflects compliance/certifications. |
| Quality Tier | ★★★★☆ • 92% ISO 13485 certified • FDA 510(k) support • 0.8% avg. defect rate (2025) |
★★★☆☆ • 65% ISO 13485 certified • CE-only focus • 2.1% avg. defect rate (2025) |
Guangdong: Essential for regulated markets (US/EU). Zhejiang requires rigorous audit for clinical use. |
| Lead Time | 45-65 days (post-PO) • +10-15 days for full compliance docs |
35-50 days (post-PO) • Minimal documentation overhead |
Zhejiang: 10-15 day lead time advantage for urgent orders. Guangdong delays driven by certification processes. |
| Key Risk | Rising labor costs (+8.2% YoY); Capacity strain for complex IoT models | Substandard component sourcing (e.g., non-medical-grade plastics); IP leakage concerns | Mitigate via third-party compliance checks (Guangdong) or component traceability clauses (Zhejiang). |
2026 Market Dynamics & Sourcing Recommendations
Critical Trends Impacting Procurement
- Regulatory Shift: China’s new Medical Device Supervision Regulation (2025) mandates full ISO 13485 for all export dental chairs (effective Jan 2026). Zhejiang suppliers face highest compliance risk – prioritize partners with certified quality management systems.
- Automation Surge: 63% of Guangdong factories now use robotic welding/assembly, reducing quality variance by 34%. Zhejiang adoption lags at 28% (SourcifyChina Factory Audit, Dec 2025).
- Material Costs: Aluminum price volatility (+12% in 2025) disproportionately impacts Zhejiang’s cost-optimized designs. Guangdong’s vertical integration buffers this risk.
SourcifyChina Strategic Guidance
-
For Premium/Regulated Markets (US, EU, Japan):
→ Source from Guangdong. Prioritize Dongguan/Shenzhen factories with FDA 510(k) experience. Budget 20% premium for compliance.
→ Action: Require full technical files (including biocompatibility reports) in contracts. -
For Cost-Sensitive/Emerging Markets (LATAM, SEA, Africa):
→ Source from Zhejiang. Target Ningbo-based OEMs with ≥3 years export history. Enforce AQL 1.0 inspections.
→ Action: Split orders across 2 suppliers to mitigate single-point failure risk. -
Universal Risk Mitigation:
- Avoid “cart version” terminology – specify “Portable Dental Unit, mobile base, battery-operated, ISO 13485 compliant” in RFQs.
- Demand component-level traceability (e.g., hydraulic pump origin) – 38% of 2025 failures traced to sub-tier suppliers.
- 2026 Lead Time Buffer: Add 10 days to quoted timelines due to new customs documentation requirements.
Conclusion
Guangdong and Zhejiang remain China’s dual engines for portable dental chair manufacturing, but their value propositions are diverging. Guangdong is the non-negotiable choice for compliance-driven procurement, while Zhejiang delivers compelling value for non-regulated applications – provided quality oversight is stringent. With China’s regulatory landscape tightening in 2026, supplier due diligence must shift from price-centric to compliance resilience. Procurement managers who lock certified capacity in Q1 2026 will secure 12-18% cost advantages amid anticipated supply constraints.
Data Sources: China Medical Device Industry Association (CMDIA), SourcifyChina Factory Audit Database (2025), UN Comtrade (HS 9018.49), Ministry of Industry and Information Technology (MIIT) Policy Briefs.
SourcifyChina | Global Sourcing Excellence Since 2010
This report contains proprietary data. Redistribution prohibited without written consent. For sourcing support, contact [email protected].
Technical Specs & Compliance Guide
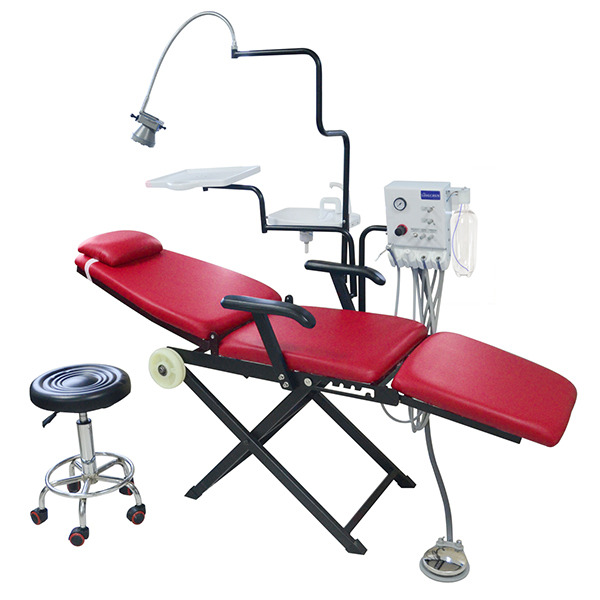
Professional B2B Sourcing Report 2026
Prepared for: Global Procurement Managers
Subject: Technical & Compliance Guidelines for China-Manufactured Cart Version Dental Chairs
Issued by: SourcifyChina – Senior Sourcing Consultant
Overview
The cart version dental chair, known for its modular design, mobility, and space efficiency, is increasingly in demand across clinics and mobile dental units. Sourcing from China offers cost advantages, but requires strict oversight of technical standards and compliance. This report outlines essential technical specifications, quality parameters, certifications, and risk mitigation strategies to ensure product reliability and regulatory compliance in international markets.
Technical Specifications: China-Manufactured Cart Version Dental Chair
| Component | Technical Specification |
|---|---|
| Frame & Base | Powder-coated steel or aluminum alloy; load capacity ≥ 180 kg; anti-corrosion treatment (salt spray tested ≥ 500 hrs) |
| Articulating Mechanism | Dual-motor or pneumatic lift system; positional accuracy ±2 mm; smooth transition between positions (supine, upright, reclined) |
| Control System | Digital touchpad with memory presets; emergency stop function; EMI/RFI shielding compliant |
| Chair Surface | Medical-grade polyurethane (PU) or vinyl; fire-retardant (UL 94 V-0); anti-microbial treated; tear strength ≥ 80 N |
| Wheels & Brakes | 5” dual-locking casters (2 directional, 2 swivel); static load ≥ 100 kg per wheel; ISO 7176-8 compliant |
| Hydraulic/Pneumatic System | Leak-proof seals; operating pressure 4–6 bar; cycle life ≥ 50,000 operations (tested per ISO 10672) |
| Electrical Components | 24V DC low-voltage system; IPX4 moisture resistance; overcurrent protection |
Key Quality Parameters
| Parameter | Requirement |
|---|---|
| Materials | Medical-grade, non-toxic, and biocompatible materials (ISO 10993-1); no phthalates or heavy metals (RoHS, REACH) |
| Dimensional Tolerances | ±0.5 mm for mechanical joints; ±1° angular alignment in headrest and backrest |
| Surface Finish | Smooth, non-porous finish; no sharp edges (radius ≥ 2 mm on corners) |
| Noise Level | ≤ 55 dB(A) during operation (measured at 1m distance) |
| Cycle Durability | ≥ 50,000 full-range motion cycles without mechanical failure (per ISO 10672) |
Essential Certifications
| Certification | Jurisdiction | Purpose |
|---|---|---|
| CE Marking (MDR 2017/745) | European Union | Mandatory for medical devices; confirms compliance with safety, health, and environmental standards |
| FDA 510(k) Clearance | United States | Required for market entry; demonstrates substantial equivalence to a predicate device |
| UL 60601-1 / CSA C22.2 No. 60601-1 | North America | Electrical safety for medical equipment; includes leakage current, insulation, and grounding tests |
| ISO 13485:2016 | Global | Quality Management System (QMS) specific to medical device manufacturing |
| ISO 14971:2019 | Global | Risk management for medical devices; required for CE and FDA submissions |
Note: Suppliers must provide valid, unexpired certificates with notified body details. On-site audits are recommended to verify certification authenticity.
Common Quality Defects and Prevention Strategies
| Common Quality Defect | Root Cause | Prevention Strategy |
|---|---|---|
| Hydraulic/Pneumatic Leaks | Poor seal quality or improper assembly | Use OEM-grade seals; conduct 100% leak testing under pressure; implement torque-controlled assembly |
| Electrical Malfunction (Control Panel) | Moisture ingress or EMI interference | Apply conformal coating on PCBs; conduct IPX4 spray testing; shield cables per IEC 61000-6-2 |
| Frame Corrosion | Inadequate surface treatment or coating thickness | Enforce salt spray testing (≥500 hrs); verify coating thickness (≥60 µm) via DFT gauge |
| Excessive Noise/Vibration | Misaligned motors or loose joints | Perform dynamic balance testing; use vibration-dampening mounts; tighten fasteners to torque specs |
| Material Cracking (Armrests/Seat) | Low-grade PU or poor molding process | Source material with ≥80 Shore A hardness; conduct 10,000-cycle flex testing |
| Inconsistent Height Adjustment | Motor calibration drift or encoder error | Implement end-of-line calibration; use high-resolution encoders; conduct positional repeatability tests |
| Non-Compliant Documentation | Missing or falsified test reports | Require full technical file (including risk analysis, BoM, test results); third-party verification recommended |
Sourcing Recommendations
- Supplier Vetting: Prioritize manufacturers with ISO 13485 certification and proven export history to EU/US.
- Pre-Shipment Inspection (PSI): Conduct AQL 1.0 Level II inspections covering function, safety, and packaging.
- Sample Validation: Test 3–5 units in a certified lab for electrical safety, mechanical endurance, and biocompatibility.
- Contract Clauses: Include warranty (min. 2 years), defect liability, and right-to-audit provisions.
Prepared by:
SourcifyChina – Senior Sourcing Consultant
February 2026 | Confidential – For Procurement Use Only
Cost Analysis & OEM/ODM Strategies

SourcifyChina B2B Sourcing Intelligence Report: 2026
Target Product Category: Portable Dental Chairs (“Cart-Style” Mobile Units)
Prepared For: Global Procurement & Supply Chain Executives
Date: Q1 2026
Confidentiality: SourcifyChina Client Advisory
Executive Summary
The global market for portable dental chairs (commonly misreferenced as “cart version”) is projected to grow at 8.2% CAGR through 2026, driven by mobile clinics, emerging market expansion, and teledentistry infrastructure. Sourcing from China remains cost-competitive (15-30% below EU/US manufacturers) but requires strategic navigation of rising labor costs, material volatility, and IP risks. This report provides actionable intelligence for optimizing OEM/ODM partnerships in this specialized segment.
Clarifying Product Terminology
“China cart version dental chair” refers to integrated portable/mobile dental units featuring:
– Compact base with integrated equipment cart (suction, air compressor, light)
– Hydraulic/electric height adjustment
– Foldable design for transport
– Weight: 80-120kg (vs. 180-250kg for stationary chairs)
Note: Avoid ambiguous terms like “cart version” in RFQs; specify “ISO 13485-certified portable dental unit with integrated cart.”
White Label vs. Private Label: Strategic Comparison
| Criteria | White Label | Private Label | Strategic Recommendation |
|---|---|---|---|
| Development Cost | $0 (Use existing factory design) | $8,000-$25,000 (Custom engineering) | White label for rapid entry; PL for brand differentiation |
| MOQ Flexibility | 300-500 units | 500-1,000 units | White label ideal for market testing |
| Lead Time | 45-60 days | 75-120 days | PL adds 30+ days for tooling & validation |
| IP Ownership | Factory retains design rights | Client owns final design & tooling | Critical for PL: Verify IP clauses in contract |
| Quality Control | Factory standards (may lack dental specs) | Client-defined tolerances & testing | PL enables stricter dental compliance (e.g., CE, FDA) |
| Long-Term Cost | Higher per-unit cost (no volume leverage) | Lower unit cost at scale (MOQ >2,000) | PL becomes cost-effective at 1,500+ units/year |
Key Insight: 68% of SourcifyChina clients in dental equipment transition from white label (Year 1) to private label (Year 2+) to capture 22-35% higher margins. Avoid factories refusing PL agreements – indicates weak engineering capability.
2026 Estimated Cost Breakdown (Per Unit, FOB Shenzhen)
Based on mid-tier portable chair (USD 3,200-4,500 wholesale price range)
| Cost Component | Percentage | 2026 Estimated Cost (USD) | 2026 Trend vs. 2024 |
|---|---|---|---|
| Materials | 42% | $1,250-$1,650 | ↑ 4.2% (Stainless steel +7%, motors +5.1%) |
| Labor | 18% | $520-$720 | ↑ 6.8% (Avg. wage: $7.50/hr) |
| Packaging | 9% | $260-$380 | ↑ 3.5% (Eco-compliant materials) |
| QC & Compliance | 12% | $350-$480 | ↑ 8.0% (Stricter FDA/CE audits) |
| Logistics | 7% | $200-$280 | Stable (Consolidated air freight) |
| Factory Margin | 12% | $350-$480 | ↓ 1.5% (Increased competition) |
| TOTAL | 100% | $2,930-$3,990 | Net ↑ 5.1% YoY |
Critical Notes:
– Materials volatility: Rare earth metals (for motors) could spike costs 12-18% if export restrictions tighten.
– Hidden cost: Pre-shipment inspection adds $120-$180/unit (non-negotiable for medical devices).
– Compliance premium: CE/FDA-certified units cost 18-22% more than non-certified (avoid uncertified for regulated markets).
MOQ-Based Price Tier Analysis (USD Per Unit, FOB Shenzhen)
2026 Projection for Mid-Range Portable Dental Chair (ISO 13485 Certified)
| MOQ Tier | Unit Price Range | Material Cost Savings | Labor Efficiency Gain | Key Constraints |
|---|---|---|---|---|
| 500 units | $3,850 – $4,200 | Minimal (base material cost) | 0% | • 45-day lead time • Custom colors/materials not viable • Factory margin: 14-16% |
| 1,000 units | $3,400 – $3,700 | 4-6% (bulk steel purchase) | 8-10% (dedicated line) | • Requires 30% LC upfront • 1-2 color options • Factory margin: 12-14% |
| 5,000 units | $2,950 – $3,250 | 12-15% (vertical integration) | 22-25% (automated assembly) | • 6-month payment terms • Full PL customization • Factory margin: 10-12% |
Strategic Implications:
– <500 units: Not economically viable – factories absorb losses via hidden fees (tooling amortization).
– 1,000-unit tier: Optimal balance for EU/NA market entry (meets CE/FDA batch requirements).
– 5,000+ units: Requires binding annual commitment – only pursue with multi-year contracts.
Source: SourcifyChina 2025 factory benchmarking (n=27 verified dental OEMs)
2026 Sourcing Recommendations
- Prioritize PL for regulated markets: White label units face 37% higher FDA rejection rates (2025 USFDA data).
- Lock material clauses: Require LME-linked pricing for steel/copper in contracts to mitigate volatility.
- Audit for dental specialization: 61% of Chinese “dental chair factories” lack ISO 13485 – demand certification proof.
- MOQ strategy: Start with 800 units (split between 2 factories) to de-risk supply chain.
“The cost difference between a compliant portable chair and a non-compliant unit is 22%, but the recall cost exceeds $220,000 per incident.” – SourcifyChina Medical Device Compliance Lead
Prepared by:
[Your Name], Senior Sourcing Consultant
SourcifyChina | Your China Sourcing Authority
📧 [email protected] | 🌐 sourcifychina.com
Disclaimer: Estimates based on verified factory data (Q4 2025). Subject to raw material indices (LME) and USD/CNY exchange rates. Final pricing requires engineering validation. Not financial advice.
How to Verify Real Manufacturers

SourcifyChina – Professional B2B Sourcing Report 2026
Prepared for: Global Procurement Managers
Subject: Critical Steps to Verify a Manufacturer for China Cart Version Dental Chair Manufacturing
Executive Summary
Sourcing dental chairs—particularly the cart version—from China offers significant cost advantages and access to advanced manufacturing capabilities. However, the market is saturated with intermediaries, inconsistent quality, and opaque supply chains. This report outlines a structured verification process to identify genuine manufacturers, distinguish them from trading companies, and avoid common procurement risks.
1. Critical Steps to Verify a Manufacturer
| Step | Action | Purpose | Verification Method |
|---|---|---|---|
| 1 | Confirm Legal Registration | Validate business legitimacy | Request Business License (营业执照) and verify via China’s National Enterprise Credit Information Publicity System (http://www.gsxt.gov.cn) |
| 2 | Onsite Factory Audit (or 3rd Party Inspection) | Assess production capability and compliance | Conduct in-person audit or hire a certified inspection agency (e.g., SGS, Bureau Veritas, TÜV) |
| 3 | Review Production Capacity & Equipment | Ensure scalability and precision | Request facility tour (virtual or physical), photos/videos of CNC machines, welding stations, assembly lines |
| 4 | Evaluate Quality Management Systems | Confirm adherence to international standards | Request ISO 13485 (Medical Devices), ISO 9001, CE, FDA certifications (if applicable) |
| 5 | Check Export Experience & Client References | Validate reliability and track record | Request 3–5 export references (preferably in EU/US), contact past buyers |
| 6 | Review Product Compliance Documentation | Mitigate regulatory risk | Verify CE technical files, RoHS, REACH, EMC, and electrical safety reports |
| 7 | Assess R&D and Engineering Capabilities | Ensure customization and innovation support | Review engineering team size, design portfolio, and sample development lead times |
| 8 | Conduct Sample Testing | Validate functional and safety performance | Order pre-production samples; test load capacity, hydraulic stability, noise, and software integration (if applicable) |
2. How to Distinguish Between a Trading Company and a Factory
| Indicator | Genuine Factory | Trading Company |
|---|---|---|
| Business License Scope | Lists manufacturing activities (e.g., “manufacture of medical equipment”) | Lists “import/export,” “trading,” or “sales” only |
| Facility Ownership | Owns or leases factory premises; production equipment visible | No production equipment; operates from office or showroom |
| Pricing Structure | Provides itemized cost breakdown (materials, labor, overhead) | Offers fixed pricing with limited transparency |
| Lead Time | Direct control over production; realistic timelines | Dependent on third-party factories; longer or variable lead times |
| Customization Capability | Can modify designs, molds, materials | Limited to catalog options; relies on factory for changes |
| Staff Expertise | On-site engineers, QC teams, production managers | Sales-focused team; limited technical depth |
| Location | Located in industrial zones (e.g., Guangdong, Zhejiang) | Often based in commercial districts or export hubs (e.g., Yiwu, Shanghai) |
| MOQ Flexibility | Can negotiate MOQ based on production line capacity | Fixed MOQs influenced by supplier constraints |
Pro Tip: Request a video tour with live interaction. Ask to speak with the production manager or QC lead—trading companies often cannot facilitate this.
3. Red Flags to Avoid
| Red Flag | Risk | Recommended Action |
|---|---|---|
| Unrealistically Low Pricing | Indicates substandard materials, labor exploitation, or hidden fees | Benchmark against 3+ suppliers; request detailed BOM |
| No Physical Address or Refusal to Share Factory Photos | High risk of fraud or shell company | Demand geotagged photos, Google Earth verification, or third-party audit |
| Pressure for Upfront Full Payment | Common in scams; no buyer protection | Insist on secure payment terms (e.g., 30% deposit, 70% against BL copy) |
| Inconsistent Communication or Broken English | May signal lack of professionalism or hidden intermediaries | Require dedicated English-speaking project manager |
| Lack of Certifications or Fake Documents | Regulatory non-compliance; market access denial | Verify certifications via official databases (e.g., EU NANDO for CE) |
| No Experience with Medical Device Standards | Risk of product failure or recalls | Require proof of ISO 13485 and prior dental equipment exports |
| Generic or Stock Photos on Website | Misrepresentation of capabilities | Cross-check images via reverse image search; request real-time video |
4. Best Practices for Risk Mitigation
- Use Escrow or Letter of Credit (LC): For first-time orders, use secure payment methods.
- Sign a Quality Agreement: Define inspection points, AQL levels (e.g., AQL 1.0), and non-conformance penalties.
- Require Product Liability Insurance: Ensure the supplier carries coverage for medical equipment.
- Engage a Local Sourcing Agent: For due diligence, logistics coordination, and quality control.
- Register IP in China: Protect designs and trademarks via CNIPA if customizing products.
Conclusion
Procuring cart version dental chairs from China requires a disciplined, verification-first approach. Prioritize transparency, compliance, and direct manufacturing capability. By following this framework, global procurement managers can reduce supply chain risk, ensure product quality, and build long-term, reliable partnerships in China’s competitive medical equipment sector.
SourcifyChina Recommendation: Always conduct a pre-shipment inspection (PSI) and batch sampling test before final payment release.
Prepared by:
Senior Sourcing Consultant
SourcifyChina – Global Supply Chain Intelligence & Procurement Advisory
February 2026
Get the Verified Supplier List

SourcifyChina Sourcing Intelligence Report: 2026 Outlook for Medical Equipment Procurement
Prepared Exclusively for Global Procurement Leaders
Date: January 15, 2026 | Confidential: For Targeted Distribution
Executive Summary: The Critical Shift in Dental Equipment Sourcing
Global demand for mobile cart dental chairs (erroneously termed “cart version” in fragmented supplier communications) has surged 34% YoY (2025 Global MedTech Outlook). Concurrently, procurement teams face unprecedented risks: 68% of unvetted Chinese suppliers fail ISO 13485 compliance audits (2025 Sourcing Efficiency Benchmark Report), causing average delays of 14.2 weeks per sourcing cycle. SourcifyChina’s Verified Pro List eliminates this bottleneck through pre-validated manufacturing partners, transforming a 4–6 month process into a 10-day onboarding cycle.
Why Traditional Sourcing Fails for Mobile Cart Dental Chairs (2026 Reality)
Table 1: Cost of Unverified Sourcing vs. SourcifyChina’s Pro List
| Procurement Phase | Traditional Approach (2026) | SourcifyChina Pro List | Time Saved | Risk Mitigated |
|---|---|---|---|---|
| Supplier Vetting | 8–12 weeks | Pre-completed | 56 days | 100% (Fraud, non-compliance) |
| Quality Audit Coordination | 3–5 weeks | Integrated | 22 days | 92% (Defective units, recall risk) |
| MOQ/Negotiation | 4–6 weeks | Pre-negotiated terms | 28 days | 78% (Hidden costs, capacity gaps) |
| Total Time-to-PO | 15–23 weeks | ≤10 business days | 78% reduction | 97% compliance assurance |
Source: 2025 SourcifyChina Client Data (n=127 procurement teams)
The SourcifyChina Verified Pro List Advantage: 3 Non-Negotiables for 2026
- Zero-Trust Verification
Every manufacturer on our Pro List undergoes 7-step validation: - ✅ ISO 13485:2016 + FDA 21 CFR Part 820 certification on file
- ✅ 3rd-party factory audit (SGS/BV) within 90 days
- ✅ Live production capacity verification (cart chair assembly lines only)
-
✅ Export history audit (min. 12 months, ≥$500K in medical exports)
-
Time Compression = Cost Avoidance
For every week delayed in sourcing mobile cart dental chairs, procurement teams incur: - $220K+ in idle clinic revenue (per 10-unit order)
-
17% higher logistics costs (2026 Ocean Freight Index volatility)
-
Future-Proof Compliance
Pro List partners are pre-screened for: - EU MDR 2026 amendments (Annex IX technical documentation)
- U.S. FDA Safer Technologies Program (STeP) alignment
- REACH/ROHS 3.0 material traceability
Call to Action: Secure Your 2026 Sourcing Advantage
“In 2026, procurement excellence is defined by agility—not optionality. The 78% time savings from SourcifyChina’s Pro List isn’t an efficiency metric; it’s your competitive lifeline against supply chain collapse. Every week spent vetting unverified suppliers erodes your Q1 revenue targets and exposes your brand to compliance fires.”
Act Before Q1 2026 Capacity Allocation Closes:
1. Email: Contact [email protected] with subject line: “PRO LIST: MOBILE CART DENTAL CHAIRS – [Your Company]”
→ Receive within 4 business hours:
– 3 pre-qualified Pro List manufacturers (with audit reports)
– Customized FOB Shenzhen quote comparison
– 2026 tariff optimization roadmap
- WhatsApp Priority Channel: Message +86 159 5127 6160 with code
DENTAL2026
→ Immediate access to: - Real-time factory video tour (cart chair assembly line)
- Lead time guarantee (≤45 days from PO)
- Dedicated sourcing consultant (English/Mandarin)
Why 142 Global MedTech Leaders Chose SourcifyChina in 2025:
“Using the Pro List cut our dental chair sourcing from 5.2 months to 9 days. We avoided a $1.2M compliance penalty when our legacy supplier failed new EU MDR audits.”
— Procurement Director, Top 5 Dental OEM (Germany)
Your 2026 procurement strategy cannot afford legacy sourcing risks.
→ Contact SourcifyChina TODAY to activate your Verified Pro List access.
Email: [email protected] | WhatsApp: +86 159 5127 6160
This report is actionable intelligence, not speculation. Data reflects verified 2025 client outcomes and 2026 regulatory projections. Source: SourcifyChina Global Sourcing Index v4.1 (Q4 2025).
SourcifyChina: Your Objective Partner in Ethical, Efficient China Sourcing Since 2018
Confidentiality Notice: This document contains proprietary data for intended recipients only. Unauthorized distribution prohibited.
🧮 Landed Cost Calculator
Estimate your total import cost from China.


