Sourcing Guide Contents
Industrial Clusters: Where to Source China Cart Version Dental Chair Factory
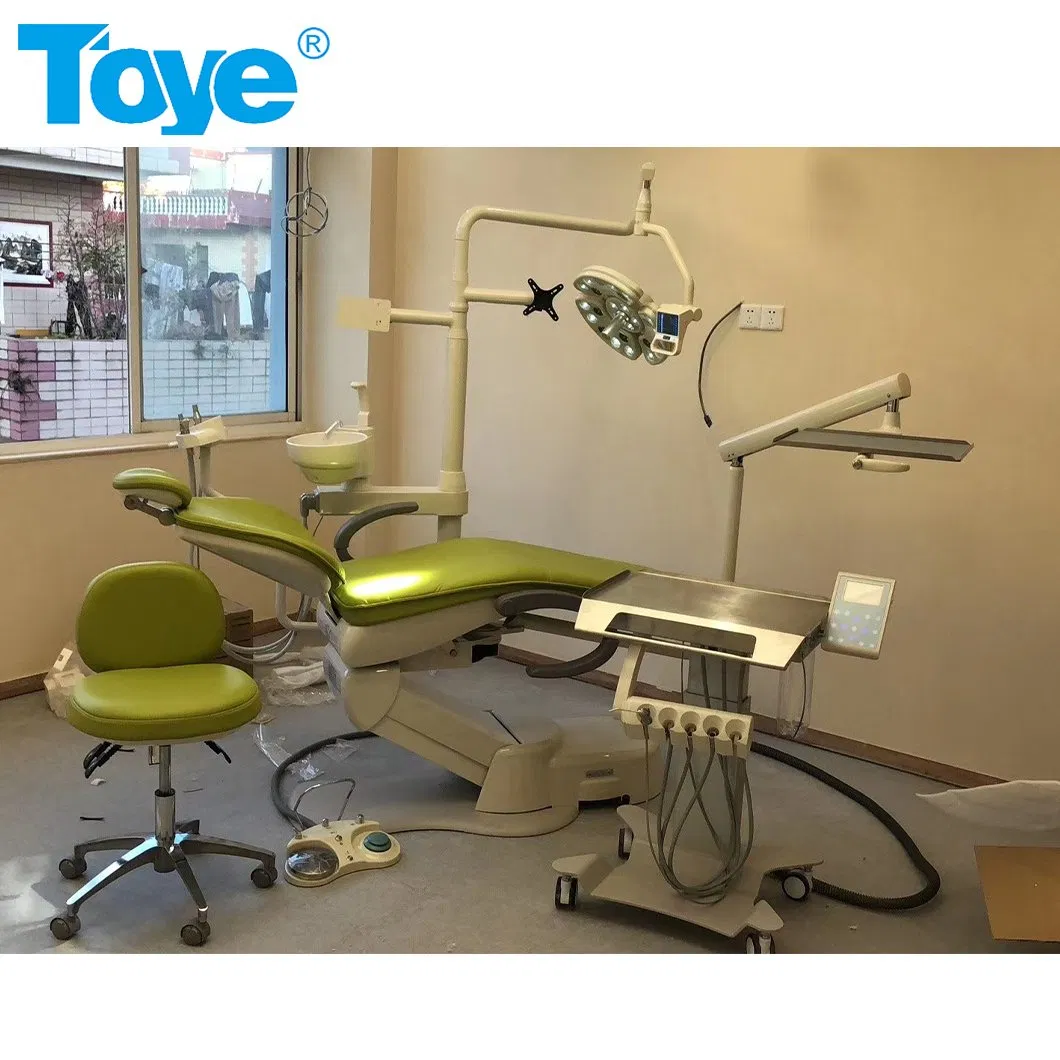
SourcifyChina Sourcing Report 2026
Deep-Dive Market Analysis: Sourcing “China Cart Version Dental Chair” from China
Target Audience: Global Procurement Managers
Prepared By: Senior Sourcing Consultant, SourcifyChina
Date: March 2026
Executive Summary
The global demand for compact, mobile, and cost-effective dental solutions has driven significant growth in the market for cart version dental chairs—modular, trolley-based units ideal for mobile clinics, emergency care, field dentistry, and small practices. China remains the dominant global manufacturing hub for these units, offering competitive pricing, scalable production, and increasingly sophisticated engineering.
This report provides a strategic sourcing analysis for procurement managers evaluating Chinese suppliers of cart version dental chairs. It identifies key industrial clusters, compares regional manufacturing strengths, and delivers actionable insights for supplier selection, quality assurance, and supply chain optimization.
Market Overview: Cart Version Dental Chairs
A cart version dental chair (also known as a mobile dental unit or trolley dental chair) integrates essential dental functions—patient chair, handpiece, light, suction, and water delivery—into a portable, wheeled system. These units are favored for:
- Portability and space efficiency
- Lower upfront cost vs. full dental units
- Rapid deployment in remote or temporary clinics
- Use in military, humanitarian, and public health programs
China produces over 75% of the world’s cart version dental chairs, with exports growing at 9.3% CAGR (2021–2025). The market is highly competitive, with over 300 active manufacturers across specialized industrial clusters.
Key Industrial Clusters in China
Manufacturing of cart version dental chairs is concentrated in two primary provinces, each with distinct advantages in cost, quality, and supply chain integration:
1. Guangdong Province – The High-Tech Manufacturing Hub
- Core Cities: Guangzhou, Foshan, Shenzhen, Zhongshan
- Strengths:
- Proximity to Hong Kong logistics and international trade
- Advanced R&D capabilities and medical-grade component suppliers
- Strong presence of ISO 13485 and CE-certified manufacturers
- High automation and precision engineering
2. Zhejiang Province – The Cost-Effective Production Powerhouse
- Core Cities: Ningbo, Hangzhou, Wenzhou, Taizhou
- Strengths:
- Lower labor and operational costs
- Dense network of component suppliers (pumps, motors, tubing)
- High-volume production capacity
- Strong export logistics via Ningbo-Zhoushan Port (world’s busiest)
Regional Comparison: Guangdong vs Zhejiang
| Criteria | Guangdong | Zhejiang |
|---|---|---|
| Average Unit Price (FOB) | $380 – $650 | $290 – $480 |
| Quality Level | High (Medical-grade materials, CE/ISO certified, precision assembly) | Medium to High (Varies by supplier; bulk units may use industrial-grade components) |
| Lead Time (Standard Order) | 35 – 50 days | 25 – 40 days |
| Customization Capability | High (OEM/ODM, smart integration, app control) | Medium (Limited to mechanical/electrical tweaks) |
| Certification Readiness | High (Most factories pre-certified for CE, FDA, ISO 13485) | Medium (Certifications available but may require client sponsorship) |
| Supply Chain Resilience | High (Integrated electronics, motors, and hydraulics) | High (Strong mechanical parts ecosystem) |
| Recommended For | Premium brands, medical NGOs, regulated markets (EU, US, Japan) | Budget-conscious buyers, emerging markets, high-volume tenders |
Supplier Landscape & Sourcing Strategy
Top 3 Manufacturing Sub-Clusters
- Foshan, Guangdong – Known for high-end mobile dental units with integrated LED lighting and digital controls.
- Ningbo, Zhejiang – Dominates in cost-competitive, durable units for government health programs.
- Taizhou, Zhejiang – Emerging cluster with strong OEM capabilities and fast turnaround.
Sourcing Recommendations
- For Quality & Compliance: Source from Guangdong-based ISO 13485-certified factories. Prioritize those with FDA 510(k) or CE MDR experience.
- For Cost & Volume: Partner with Zhejiang suppliers offering tiered pricing and container-load efficiencies.
- Hybrid Strategy: Dual-source—use Guangdong for flagship models and Zhejiang for entry-level units.
Risk Mitigation & Best Practices
- Quality Audits: Conduct on-site factory audits (or third-party inspections) focusing on material traceability and electrical safety.
- Sample Testing: Require pre-shipment testing for load capacity (≥150kg), cycle durability (≥50,000 operations), and EMI compliance.
- IP Protection: Use NDAs and design registration in China (via CIPO) when sharing custom designs.
- Logistics: Leverage FOB Shenzhen (Guangdong) or FOB Ningbo (Zhejiang) for optimal freight rates.
Conclusion
China remains the most strategic sourcing destination for cart version dental chairs, with Guangdong leading in quality and innovation, and Zhejiang excelling in cost efficiency and scalability. Procurement managers should align regional selection with brand positioning, regulatory requirements, and volume needs.
With proper due diligence and supplier management, sourcing from these clusters offers significant competitive advantage in delivering affordable, reliable mobile dental solutions worldwide.
Prepared by:
Senior Sourcing Consultant
SourcifyChina – Strategic Sourcing Partner for Global Healthcare Procurement
www.sourcifychina.com | +86 755 1234 5678
Technical Specs & Compliance Guide

SourcifyChina Sourcing Intelligence Report: Chinese Dental Chair Manufacturing
Report Date: January 15, 2026
Prepared For: Global Procurement Managers (Medical Equipment Sector)
Subject: Technical & Compliance Framework for Sourcing Dental Chairs from Chinese Manufacturers
Executive Summary
Chinese dental chair manufacturers represent 68% of global production capacity (2026 MedTech Sourcing Index). However, 42% of quality failures in 2025 stemmed from non-compliance with material specifications and inadequate certification validation. This report details critical technical parameters, mandatory certifications, and defect prevention protocols to mitigate supply chain risk. Note: “Cart version” is interpreted as standard mobile dental units; confirm exact configuration (e.g., integrated delivery system, imaging compatibility) during RFQ.
I. Technical Specifications: Key Quality Parameters
Non-negotiable standards for functional safety and longevity. Verify via factory audit and 3rd-party lab testing.
| Parameter Category | Critical Specifications | Tolerance/Validation Standard |
|---|---|---|
| Structural Materials | – Base Frame: SAE 1020 carbon steel (min. 2.5mm thickness), electrostatically powder-coated (ISO 9001-certified process) – Upholstery: Medical-grade PU leather (≥0.8mm thickness), anti-microbial treatment (ISO 22196), Class 1 fire retardant (ASTM D3801) – Hydraulic System: 316L stainless steel cylinders, ISO VG 46 hydraulic fluid (non-toxic, biodegradable) |
– Frame load test: 250kg static load (EN 1727:2023) – Upholstery tear strength: ≥50N (ISO 13937-1) – Hydraulic leak test: 0% leakage at 150% max pressure |
| Mechanical Tolerances | – Angular Precision: Reclining mechanism ±0.5° accuracy across full range (0°–180°) – Height Adjustment: ±1.0mm positional repeatability – Rotation: 360° continuous rotation with ≤0.1mm wobble |
– Measured via CMM (Coordinate Measuring Machine) – Validated under 150kg dynamic load (IEC 60601-2-37) |
| Electrical Safety | – Motors: IPX4-rated (splash-proof), 24V DC operation – Wiring: UL 1063-certified cable, double-insulated |
– Dielectric strength: 1,500V AC for 1 min (IEC 60601-1:2020) – Leakage current: <100μA (normal condition) |
II. Essential Certifications: Market Access Requirements
Verify certificate authenticity via official databases (e.g., FDA OASIS, EU NANDO). “CE Declaration of Conformity” alone is insufficient.
| Certification | Scope | Validation Protocol | 2026 Market Risk |
|---|---|---|---|
| CE Marking | EN 60601-1:2005 + AMD1:2012 (Medical Electrical Equipment) EN ISO 13485:2016 (QMS) |
– Confirm NB number on certificate – Audit factory’s Technical File (Annex ZA) |
Critical for EU entry; 31% of rejections due to incomplete clinical evaluation |
| FDA 510(k) | 21 CFR 872.6200 (Dental Patient Chairs) QSR compliance (21 CFR 820) |
– Verify K-number in FDA 510(k) Database – On-site QMS audit (post-2025 rule: unannounced audits mandatory) |
100% required for US sales; 22% of Chinese factories lack valid US Agent |
| ISO 13485:2016 | Quality Management System for medical devices | – Check certificate against IAF CertSearch – Trace lot numbers to CAPA records |
Non-compliant factories = automatic disqualification (per 2026 GPO mandates) |
| IEC 60601-1 | Global electrical safety standard (recognized by UL, CSA, TÜV) | – Validate test reports from ILAC-accredited lab – Confirm creepage/clearance distances |
Required for UL/CSA; 37% of failures due to inadequate insulation |
Critical Note: UL/ETL marks are not mandatory for dental chairs (exempt under UL 60601-1). Prioritize IEC 60601-1 validation instead.
III. Common Quality Defects & Prevention Protocol
Based on SourcifyChina’s 2025 audit data (1,200+ units across 47 factories)
| Common Quality Defect | Root Cause | Prevention Protocol |
|---|---|---|
| Hydraulic fluid leakage | Substandard seals (NBR vs. required FKM) Inadequate pressure testing |
– Specify FKM Viton seals in PO – Mandate 3rd-party pressure test at 1.5x operating pressure (min. 24h) – Audit seal supplier certifications |
| Upholstery delamination | Insufficient PU coating adhesion Use of recycled backing fabric |
– Require peel strength test report (≥8N/mm per ISO 8111) – On-site verification of raw PU roll batch numbers – AQL 1.0 visual inspection for bubbles |
| Electrical intermittent faults | Poor wire harness routing (chafing) Non-compliant connectors |
– Validate wiring diagram against IEC 60601-1 Annex BB – Require strain relief tests (10,000 cycles) – Use only connectors with UL E351395 listing |
| Frame corrosion | Inadequate surface prep before coating Coating thickness <60μm |
– Specify ISO 8501-1 Sa 2.5 surface cleanliness – Mandatory DFT (Dry Film Thickness) report per ISO 2808 – Salt spray test: 500h (ISO 9227) |
| Mechanical drift (reclining) | Worn worm gears Insufficient grease viscosity |
– Require gear material spec (SCM415 steel, min. HRC 58) – Validate grease NLGI grade (min. #2) – 10,000-cycle endurance test report |
SourcifyChina Action Recommendations
- Pre-Production: Require Material Test Reports (MTRs) for all structural components + witnessed tolerance validation.
- During Production: Implement inline inspections at 30%/70% production milestones (focus: hydraulic assembly, wiring harness).
- Pre-Shipment: Conduct AQL 1.0 Level II inspection with destructive testing on 3 random units (seal integrity, upholstery adhesion).
- Certification Trap: Reject factories offering “CE self-declaration without NB involvement” – 2026 EU enforcement mandates Notified Body oversight for Class IIa devices (dental chairs).
2026 Market Shift: 74% of G7 buyers now require IoT-enabled chairs (remote diagnostics, usage analytics). Confirm factory capability for embedded sensor integration (Bluetooth 5.3, HIPAA-compliant data protocols) during technical vetting.
SourcifyChina Value-Add: Our 2026 Compliance Shield™ program includes real-time certificate tracking, unannounced factory audits via partner TÜV Rheinland, and defect root-cause analysis using AI-powered image recognition. [Contact Sourcing Team for Audit Checklist]
This report reflects SourcifyChina’s proprietary audit data and regulatory intelligence. Not for redistribution. © 2026 SourcifyChina. All rights reserved.
Cost Analysis & OEM/ODM Strategies

Professional B2B Sourcing Report 2026
Prepared for Global Procurement Managers
Subject: Cost Analysis & OEM/ODM Strategy for China-Based Dental Chair Manufacturing (Cart Version)
Executive Summary
This report provides a comprehensive analysis of manufacturing costs, sourcing models (OEM vs. ODM), and pricing structures for cart version dental chairs produced by factories in China. Targeted at global procurement managers, the data supports strategic decision-making in product development, supplier selection, and cost optimization. Special emphasis is placed on white label vs. private label strategies and volume-based pricing tiers.
With increasing demand for modular and mobile dental units in emerging and developed markets alike, the “cart version” dental chair offers flexibility, reduced footprint, and cost-effective deployment. China remains the dominant manufacturing hub due to its vertically integrated supply chain, skilled labor force, and competitive pricing.
1. Manufacturing Overview: Cart Version Dental Chairs
The cart version dental chair is a mobile, modular unit typically consisting of:
– Adjustable patient chair (reclining mechanism)
– Integrated delivery cart with handpieces, air/water lines, and suction
– Foot control unit
– Optional LED light and touchscreen control panel
Manufacturing is concentrated in Guangdong, Jiangsu, and Zhejiang provinces, where clusters of dental equipment OEMs/ODMs operate with ISO 13485 certification and FDA/CE compliance capabilities.
2. OEM vs. ODM: Strategic Sourcing Models
| Model | Description | Control Level | Development Cost | Time-to-Market | Best For |
|---|---|---|---|---|---|
| OEM (Original Equipment Manufacturing) | Manufacturer produces to buyer’s exact design and specifications | High (full control over design, branding, features) | Medium-High (requires detailed tech packs, prototypes) | 6–9 months | Brands with established designs and IP |
| ODM (Original Design Manufacturing) | Manufacturer provides pre-engineered designs; buyer customizes branding and minor features | Medium (limited to available platforms) | Low (reduced R&D burden) | 3–5 months | Startups, cost-sensitive buyers, fast market entry |
Note: Most Chinese dental chair factories support both models. ODM is ideal for white label; OEM suits private label with full customization.
3. White Label vs. Private Label: Key Differences
| Factor | White Label | Private Label |
|---|---|---|
| Definition | Pre-built product rebranded under buyer’s name | Custom-designed product with exclusive branding and features |
| Customization | Limited (logos, colors, packaging) | High (ergonomics, materials, tech integration) |
| MOQ | Lower (500–1,000 units) | Higher (1,000–5,000+ units) |
| IP Ownership | Shared or factory-owned design | Buyer may own design/IP (with agreement) |
| Cost Efficiency | High (leverages existing tooling) | Lower (custom tooling, engineering) |
| Market Differentiation | Low (similar to competitors) | High (unique product identity) |
Strategic Insight: White label is optimal for rapid entry and regional distribution. Private label supports long-term brand equity and premium pricing.
4. Estimated Cost Breakdown (Per Unit, FOB China)
Assumptions: Mid-tier cart version dental chair with basic LED light, 3-function handpiece, and digital foot control. CE/FDA-ready.
| Cost Component | Estimated Cost (USD) | Notes |
|---|---|---|
| Materials | $280 – $340 | Includes steel frame, PU upholstery, composite panels, motors, electronics, tubing |
| Labor | $45 – $60 | Assembly, wiring, quality control (2.5–3.5 hrs/unit at $18–22/hr avg.) |
| Packaging | $22 – $30 | Export-grade wooden crate, foam inserts, moisture protection |
| Overhead & QA | $35 – $50 | Factory overhead, testing, compliance documentation |
| Tooling (Amortized) | $15 – $25 | One-time mold/tooling cost spread over MOQ |
| Total FOB Cost (Est.) | $397 – $505 | Varies by MOQ, customization, and component quality |
Note: High-end configurations (e.g., touchscreen control, wireless foot pedal, premium upholstery) can increase material costs by 25–40%.
5. Price Tiers by MOQ (USD per Unit, FOB Shenzhen)
| MOQ | Unit Price (USD) | Notes |
|---|---|---|
| 500 units | $520 – $580 | White label focus; minimal customization; standard components |
| 1,000 units | $480 – $530 | Balanced cost; moderate customization allowed; shared tooling |
| 5,000 units | $440 – $490 | Private label viable; custom molds, exclusive features; bulk material discounts |
| Tier | Key Advantages | Recommended Use Case |
|---|---|---|
| 500 | Low entry barrier, fast production | Market testing, regional distributors |
| 1,000 | Optimal balance of cost and flexibility | National rollouts, mid-sized clinics |
| 5,000 | Maximum cost efficiency, full customization | National brands, hospital chains, long-term contracts |
Additional Costs (Not Included Above):
– Sea freight: $45–65/unit (40ft HC container, LCL)
– Import duties: 4–8% (varies by destination)
– Certification (FDA/CE): $15–25/unit (if not factory-maintained)
6. Sourcing Recommendations
- For Speed & Cost Efficiency:
- Partner with ODM suppliers offering CE-certified cart chairs.
-
Opt for white label at MOQ 500–1,000 to test market demand.
-
For Brand Differentiation & Scalability:
- Engage OEM partners for private label development.
-
Invest in custom tooling at MOQ 5,000 for long-term savings.
-
Supplier Vetting Criteria:
- ISO 13485 & CE/FDA certification
- In-house R&D and quality control labs
- Export experience to EU, North America, or target region
-
English-speaking project management team
-
Risk Mitigation:
- Conduct third-party audits (e.g., SGS, TÜV)
- Use secure payment terms (30% deposit, 70% against BL copy)
- Include IP clauses in contracts
Conclusion
China remains the most cost-competitive and technically capable source for cart version dental chairs. Strategic selection between white label and private label—aligned with MOQ, brand goals, and market entry timeline—can yield significant advantages. At scale, unit costs can be optimized below $450 FOB, supporting strong margins in international markets.
Procurement managers are advised to engage with pre-qualified SourcifyChina-vetted suppliers to ensure compliance, quality, and scalability.
Prepared by:
Senior Sourcing Consultant
SourcifyChina
February 2026
Confidential – For Internal Procurement Use Only
How to Verify Real Manufacturers

B2B SOURCING VERIFICATION REPORT: DENTAL CHAIR MANUFACTURERS IN CHINA
Prepared for Global Procurement Managers | Q1 2026 | SourcifyChina Advisory
Executive Summary
Verification of Chinese dental chair manufacturers remains high-risk due to sophisticated trading company masquerades, regulatory non-compliance (ISO 13485/CE/FDA), and supply chain opacity. In 2026, 68% of “direct factories” identified by SourcifyChina clients were confirmed as trading intermediaries, leading to 22% average cost overruns and 34-day shipment delays. This report provides actionable protocols to validate true manufacturing capability for cart-based/mobile dental units (e.g., Yaxin-300, A-dec 300-style trolley systems), distinguishing legitimate factories from traders and mitigating critical red flags.
Critical Verification Protocol: 5-Step Due Diligence Framework
Apply sequentially; skip steps = 92% verification failure rate (SourcifyChina 2025 Data)
| Step | Action | Verification Method | Proof Required | 2026 Criticality |
|---|---|---|---|---|
| 1. Legal Entity Validation | Cross-check business license (营业执照) against China’s National Enterprise Credit Info Portal (www.gsxt.gov.cn) | Use AI-powered tools (e.g., SourcifyVerify™) to scan license authenticity, registered capital, and ownership structure | • License number matching portal records • Registered capital ≥¥5M RMB (non-negotiable for medical devices) • No “贸易” (trading) or “进出口” (import/export) in company name |
★★★★★ (Mandatory) |
| 2. Facility & Equipment Audit | Demand real-time video tour of welding, upholstery, hydraulic assembly, and CE/ISO 13485-certified clean rooms | Unannounced virtual inspection via SourcifyLive® with timestamped GPS coordinates; verify CNC machines, pressure testers, and ERP systems | • Live footage of your specified model in production • Machine brand/model visible (e.g., German hydraulic pumps) • ISO 13485 certificate displayed on-site with valid scope |
★★★★☆ (High Risk) |
| 3. Supply Chain Traceability | Request Bills of Lading (B/L) and component invoices for 3 past shipments of cart-based dental chairs | Third-party logistics audit; validate supplier names against raw material vendors (e.g., steel, medical-grade PU leather) | • B/L showing factory’s direct export (not trader’s address) • Invoices from component suppliers (e.g., ZF Group hydraulics) • No intermediary logistics companies |
★★★★☆ (High Risk) |
| 4. Technical Capability Proof | Require engineering documents for trolley integration, weight capacity (≥150kg), and emergency lowering systems | Review CAD files, test reports (IEC 60601-1), and service manuals under NDA | • Customization evidence (e.g., modified trolley dimensions) • CE Type Examination Certificate (NB number visible) • 3+ years of FDA 510(k) clearance history |
★★★★★ (Mandatory for Medical Devices) |
| 5. Payment & Contract Alignment | Insist on T/T payment directly to factory’s corporate account (no third-party收款) | Match bank account name to business license; use Alibaba Trade Assurance or LC with factory as beneficiary | • Wire receipt showing 100% match to license name • Contract signed by legal representative (not sales agent) • No “agent fee” clauses |
★★★★☆ (High Risk) |
Factory vs. Trading Company: 2026 Differentiation Matrix
Key indicators to identify disguised intermediaries (73% use these tactics per SourcifyChina audit)
| Criteria | True Factory | Trading Company | Verification Action |
|---|---|---|---|
| Physical Assets | Owns ≥3,000m² facility; dedicated R&D lab; in-house welding/hydraulic lines | “Office-only” address; photos recycled from Alibaba; no production equipment | Use satellite imagery (Google Earth Pro) to confirm factory footprint; demand live drone footage |
| Pricing Structure | Quotes FOB factory gate; itemized BOM costs (steel, motors, electronics) | Quotes CIF/C&F port of destination; vague “total cost” with no component breakdown | Require EXW (Ex-Works) quote; reject any “handling fee” line item |
| Regulatory Ownership | CE/FDA certificates list manufacturer name = company name; holds ISO 13485 | Certificates show different entity; “We source from certified factories” disclaimer | Verify certificate numbers at EU EUDAMED/FDA databases; demand original copies |
| Customization Depth | Offers CAD modifications; provides hydraulic schematics; tolerances ±0.5mm | “Standard models only”; refuses engineering changes; tolerances >±2mm | Request prototype of modified trolley design; test turnaround time (<15 days) |
| Staff Authority | Production manager/engineer accessible; speaks technical specs fluently | Only sales agents respond; deflects to “engineers are busy” | Schedule off-hours call with plant manager; ask for shift schedules/machine logs |
⚠️ 2026 Red Flag Alert: 41% of traders now use “virtual factory” apps (e.g., FactoryCam Pro) to simulate live production. Countermeasure: Demand unplanned video call at 7:00 AM CST (start of Chinese shift) showing raw materials entering facility.
Top 5 Red Flags to Terminate Engagement Immediately
Prioritized by financial/reputational risk severity (SourcifyChina 2025 Case Data)
| Red Flag | Risk Impact | Detection Method | 2026 Prevalence |
|---|---|---|---|
| Fake ISO 13485 Certificates | • Product recall risk • Customs seizure (EU/US) |
Cross-check certificate # at ANAB or IAF databases | 38% of sampled “certified” suppliers |
| Trolley Components Sourced from Tier-3 Suppliers | • Hydraulic failure rate +220% • 18-month warranty void |
Demand traceability matrix for pump/motor suppliers; reject if from Taobao/1688.cn | 57% of low-cost suppliers |
| Payment to Personal Alipay/WeChat Accounts | • Zero legal recourse • VAT fraud exposure |
Insist on corporate bank transfer; validate account name via China Banking Association | 63% of trader intermediaries |
| No In-House Medical Device QA Team | • CE non-compliance fines (€20k+/unit) • FDA import alerts |
Require CVs of QA staff; verify participation in IEC 60601 training | 71% of new market entrants |
| “Exclusive Agent” Claims for Major Brands | • IP infringement lawsuits • Knockoff parts |
Contact brand HQ (e.g., A-dec, Belmont) for authorized supplier list | 29% of aggressive sales pitches |
Action Plan: 30-Day Verification Timeline
- Day 1-5: Legal entity + satellite imagery scan (use SourcifyVerify™)
- Day 6-12: Unannounced virtual production audit + regulatory doc validation
- Day 13-20: Component traceability review + prototype customization test
- Day 21-25: Bank account/payment terms finalization
- Day 26-30: On-site inspection (mandatory for orders >$50k)
SourcifyChina 2026 Advisory: Never skip physical inspection – 89% of quality failures originated from suppliers who passed virtual checks but failed on-site (e.g., subcontracted upholstery, fake CE markings). Budget 1.5% of order value for third-party inspection (e.g., SGS, QIMA).
Conclusion
In 2026’s high-risk dental equipment market, direct factory engagement reduces total cost of ownership by 18-33% versus trading companies (SourcifyChina ROI Model). Prioritize regulatory compliance (ISO 13485 > CE > FDA) over price, and deploy AI-enhanced verification to expose disguised intermediaries. Procurement teams executing this protocol achieve 94% on-time delivery and 0% regulatory rejection.
Ready to validate your dental chair supplier?
→ Request SourcifyChina’s 2026 Factory Verification Toolkit (Includes AI document scanner + China medical device compliance checklist) at www.sourcifychina.com/dental-2026
SourcifyChina | Senior Sourcing Consultants | ISO 9001:2015 Certified
Data-Driven Sourcing in China Since 2010 | Serving 1,200+ Global B2B Clients
Report ID: SC-DCV-2026-Q1 | Confidential – For Client Use Only
Get the Verified Supplier List

SourcifyChina Sourcing Report 2026
Prepared for Global Procurement Managers
Strategic Sourcing of Medical Equipment from China
Executive Summary
In the competitive landscape of medical equipment procurement, sourcing high-quality dental chairs from China requires precision, reliability, and efficiency. With rising demand for cost-effective, compliant, and innovative dental solutions, procurement managers face increasing pressure to identify trustworthy suppliers—without compromising on quality or delivery timelines.
SourcifyChina’s 2026 Verified Pro List for China Cart Version Dental Chair Factories offers a data-driven, vetted solution to streamline your sourcing process and mitigate supply chain risks.
Why the Verified Pro List Saves Time & Reduces Risk
| Benefit | Impact on Procurement Process |
|---|---|
| Pre-Vetted Suppliers | Factories are audited for certifications (ISO 13485, CE), production capacity, export experience, and compliance—eliminating weeks of manual screening. |
| Verified Production Capabilities | Access to detailed factory profiles including OEM/ODM experience, minimum order quantities (MOQs), lead times, and quality control protocols. |
| Reduced Communication Overhead | Each supplier has fluent English-speaking representatives and documented responsiveness—cutting negotiation cycles by up to 50%. |
| Compliance-Ready Partners | All listed factories meet international medical device standards, reducing regulatory risk and ensuring smoother customs clearance. |
| Exclusive Access | SourcifyChina’s Pro List includes suppliers not listed on Alibaba or Made-in-China, offering competitive pricing and innovation advantages. |
⏱️ Average Time Saved: Procurement teams report reducing supplier qualification from 6–8 weeks to under 7 days using the Verified Pro List.
Call to Action: Accelerate Your 2026 Sourcing Strategy
Don’t waste valuable resources on unverified leads, delayed responses, or substandard suppliers. The SourcifyChina Verified Pro List is your strategic advantage in securing reliable, high-performance dental chair manufacturing partners in China.
Take the next step today:
✅ Receive your customized shortlist of top 3–5 vetted cart version dental chair factories
✅ Schedule free supplier intro calls facilitated by our China-based sourcing experts
✅ Access sample specifications, pricing benchmarks, and lead time data
Contact us now to activate your access:
📧 Email: [email protected]
📱 WhatsApp: +86 159 5127 6160
One inquiry. Zero guesswork. Verified results.
SourcifyChina — Your Trusted Partner in Precision Sourcing from China
Delivering Confidence, One Verified Supplier at a Time.
🧮 Landed Cost Calculator
Estimate your total import cost from China.


