The global calcium stearate market is experiencing steady growth, driven by rising demand across industries such as plastics, pharmaceuticals, construction, and food processing. According to Grand View Research, the global calcium stearate market size was valued at approximately USD 554.8 million in 2022 and is projected to expand at a compound annual growth rate (CAGR) of over 5.2% from 2023 to 2030. This growth is fueled by increasing applications as a lubricant, release agent, and stabilizer in polymer production, alongside growing infrastructure development and pharmaceutical manufacturing in emerging economies. With supply chain dynamics and product quality becoming critical differentiators, identifying reliable manufacturers is essential for downstream industries requiring consistent performance and regulatory compliance. Based on production capacity, global reach, industry reputation, and technological capabilities, the following seven companies stand out as leading calcium stearate manufacturers shaping the current market landscape.
Top 7 Calcium Sterate Manufacturers 2026
(Ranked by Factory Capability & Trust Score)
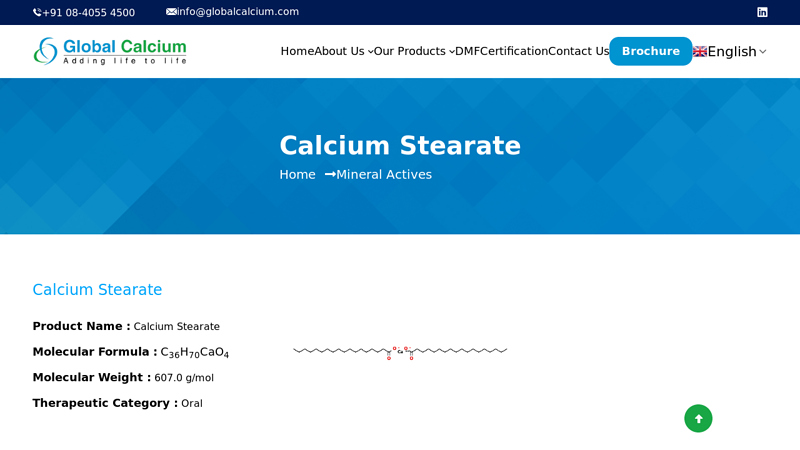
#1 Calcium Stearate
Domain Est. 2000
Website: globalcalcium.com
Key Highlights: Global Calcium is one of the leading manufacturers and exporters of Calcium Stearate. We support the customers with exhaustive documentation….
#2 Calcium-Stearate-NF
Domain Est. 1995
Website: spectrumchemical.com
Key Highlights: 15-day returnsCalcium-Stearate-NF at Spectrum Chemical. Manufactured, packaged and stored under current Good Manufacturing Practices (cGMP) per 21CFR part 211 in FDA ……
#3 NSF Product and Service Listings
Domain Est. 1996
Website: info.nsf.org
Key Highlights: Calcium Stearate+ [1], RSN Powder Hydense (Code 5870), 2.5 ; Calcium Stearate+ [1], Standard Hydense (Code 5871), 2.5 ; + Material complies with NSF/ANSI/CAN 61 ……
#4 CALCIUM STEARATE
Domain Est. 1997
Website: blachford.com
Key Highlights: CALCIUM STEARATE L-155, L-155V, 325, and COARSE are fusion-reacted calcium stearates suitable for FDA 21 CFR indirect food contact applications….
#5 Calcium Stearate
Domain Est. 1998
Website: allanchem.com
Key Highlights: Allan Chemical Corporation provides kosher-certified calcium stearate that complies with both USP and FCC standards. Each product is backed by ……
#6 Calcium Stearate Supplier
Domain Est. 2000
Website: vivion.com
Key Highlights: Need a dependable wholesale Calcium Stearate Supplier? Choose Vivion, the leading distributor of bulk ingredients and chemicals. Improve formulations today….
#7 Calcium Stearate
Website: tepekimya.com.tr
Key Highlights: Calcium stearate (Ca-30) is a neutral calcium soap manufactured from a high-quality vegetable source of stearic acid….
Expert Sourcing Insights for Calcium Sterate
H2 2026 Market Trends Analysis for Calcium Stearate
The Calcium Stearate market in the second half of 2026 is expected to be shaped by a confluence of factors driven by downstream industries, raw material dynamics, sustainability pressures, and regional economic conditions. Here’s a breakdown of the key trends anticipated:
-
Sustained Demand from Key Industries, with Shifting Focus:
- Construction & PVC Stabilization: Demand will remain robust, particularly in emerging markets experiencing infrastructure growth (Asia-Pacific, parts of Africa, Latin America). However, the trend towards lead-free stabilizers in PVC will solidify, making calcium stearate (often paired with zinc stearate) a standard solution, especially in pipe, profile, and cable applications. Growth will be steady but potentially moderated by overall construction sector fluctuations.
- Pharmaceuticals & Cosmetics: This high-value segment will see consistent demand. Calcium stearate’s role as a lubricant in tablet manufacturing and a thickening/stabilizing agent in cosmetics will be stable. Innovation in drug delivery systems and premium personal care products could provide incremental growth, but regulatory scrutiny remains paramount.
- Food Industry: Demand will be stable but highly regulated. Its use as an anti-caking agent in powdered foods (e.g., spices, baking mixes) will continue, but growth is limited by strict food-grade specifications and competition from alternatives like silica.
- Rubber & Plastics (Beyond PVC): Use as a lubricant and release agent in rubber processing (tires, technical goods) and other plastics (polyolefins, engineering plastics) will persist. Demand will correlate closely with automotive and industrial manufacturing output.
-
Raw Material Price Volatility and Supply Chain Resilience:
- Stearic Acid Dependency: Calcium stearate production is heavily reliant on stearic acid, derived from tallow (animal fat) or palm oil. H2 2026 will likely see continued volatility:
- Tallow: Prices influenced by livestock cycles, biofuel demand (especially for used cooking oil competing with tallow), and global meat consumption patterns.
- Palm Oil: Prices remain sensitive to weather (El Niño/La Niña impacts), Indonesian/Malaysian government policies (taxes, export bans), and global vegetable oil demand (food, biofuels). Sustainability concerns (deforestation) could lead to premiums for certified sustainable palm oil (CSPO), impacting costs.
- Supply Chain Focus: Manufacturers will prioritize securing long-term contracts with stearic acid suppliers and diversifying sources (geographic and feedstock – tallow vs. palm) to mitigate volatility. Nearshoring or regional production hubs may gain traction to reduce logistics risks.
- Stearic Acid Dependency: Calcium stearate production is heavily reliant on stearic acid, derived from tallow (animal fat) or palm oil. H2 2026 will likely see continued volatility:
-
Intensifying Focus on Sustainability and Green Chemistry:
- Bio-based & Renewable Claims: Expect increased marketing emphasis on calcium stearate derived from sustainable palm oil (RSPO certified) or tallow (considered a co-product/waste stream). “Bio-based” labeling will become more common.
- Regulatory Pressure: Regulations like REACH (EU) and evolving chemical safety frameworks globally will maintain pressure for transparency in sourcing and manufacturing processes. Life Cycle Assessments (LCAs) may become more important for large buyers.
- Circular Economy: Interest in utilizing waste fats and greases as feedstock for stearic acid (and thus calcium stearate) could grow, driven by circular economy goals, though technical challenges remain.
-
Regional Market Divergence:
- Asia-Pacific: Expected to remain the largest and fastest-growing market, driven by massive construction activity in India, Southeast Asia, and ongoing industrialization. China’s demand will be significant but potentially more mature and influenced by its own economic stimulus measures.
- North America & Europe: Growth will be more moderate, focused on replacement demand, compliance with regulations (e.g., lead-free PVC), and innovation in pharma/cosmetics. Sustainability requirements will be most stringent here.
- Emerging Markets (Africa, Latin America): Representing significant growth potential, particularly in construction and basic manufacturing, but growth will be sensitive to local economic stability, infrastructure, and foreign investment.
-
Competitive Landscape and Innovation:
- Consolidation: Potential for further consolidation among producers seeking economies of scale and geographic reach to better manage raw material costs and serve global clients.
- Product Differentiation: Focus on high-purity grades for pharma/cosmetics, specialized particle sizes for specific applications, and blends with other lubricants/stabilizers (e.g., Ca/Zn systems) will be key differentiators.
- Efficiency Improvements: Producers will continue investing in process optimization to improve yield, reduce energy consumption, and minimize waste, partly driven by cost pressures and sustainability goals.
Summary Outlook for H2 2026:
The Calcium Stearate market in H2 2026 is projected for moderate, steady growth globally, underpinned by its essential role in PVC stabilization and key industrial processes. Growth will be strongest in emerging economies, particularly in Asia-Pacific construction. The market will be significantly influenced by ongoing volatility in stearic acid prices (linked to palm oil and tallow markets) and increasing emphasis on sustainability in sourcing and manufacturing. Producers who can secure reliable, sustainable feedstocks, ensure supply chain resilience, and offer differentiated, high-quality products will be best positioned to navigate the challenges and capitalize on the opportunities in the latter half of 2026.

Common Pitfalls in Sourcing Calcium Stearate (Quality, IP)
Sourcing Calcium Stearate effectively requires careful attention to both quality consistency and intellectual property (IP) considerations. Overlooking these aspects can lead to significant operational, legal, and reputational risks.
Quality Pitfalls
Inconsistent Raw Material Sources
Calcium stearate quality heavily depends on the source and purity of the stearic acid used. Suppliers sourcing stearic acid from mixed animal/vegetable origins or varying geographical regions may deliver batches with inconsistent fatty acid profiles. This variability affects critical performance attributes like melting point, lubricity, and dispersion in PVC or rubber formulations, leading to processing issues or final product defects.
Inadequate Purity and Contaminant Control
Low-grade calcium stearate may contain excessive free stearic acid, moisture, or inorganic impurities (e.g., residual catalysts like lead or arsenic from outdated production methods). These contaminants can degrade polymer stability, cause plate-out in extrusion, or violate regulatory standards (e.g., REACH, FDA for food-contact applications), potentially resulting in product recalls.
Lack of Batch-to-Batch Consistency
Suppliers without robust quality management systems (e.g., ISO 9001) may fail to maintain consistent particle size distribution, bulk density, or calcium content. Inconsistent physical properties impair dosing accuracy in automated processes and compromise product reproducibility, especially in sensitive applications like pharmaceuticals or high-performance plastics.
Insufficient Documentation and Testing
Accepting certificates of analysis (CoA) without verifying testing methodologies or audit trails is risky. Some suppliers may provide incomplete CoAs lacking data on heavy metals, peroxide value, or acid number. Without access to batch-specific test reports or third-party verification, buyers cannot confirm compliance with technical or regulatory requirements.
Intellectual Property (IP) Pitfalls
Unlicensed Use of Proprietary Formulations
Calcium stearate is often incorporated into patented compound formulations (e.g., specialty lubricants, medical devices). Sourcing generic material without assessing downstream IP obligations can inadvertently infringe on formulation patents. Buyers may become liable if their use of the sourced calcium stearate enables the manufacture of a protected end-product.
Supplier IP Ownership Ambiguity
Contracts that fail to clarify IP rights in custom-developed grades (e.g., surface-modified or nano-dispersed calcium stearate) may leave buyers without ownership or usage rights. If a supplier retains IP, they could restrict supply, increase prices, or license the innovation to competitors, undermining the buyer’s competitive advantage.
Reverse Engineering Risks
Sourcing from low-cost suppliers in jurisdictions with weak IP enforcement increases the risk of obtaining material produced via reverse-engineered processes protected by patents. Using such material exposes the buyer to contributory infringement claims, particularly in regulated industries like pharmaceuticals or electronics.
Inadequate IP Due Diligence in Supplier Selection
Failing to evaluate a supplier’s own IP portfolio—such as patents on production processes (e.g., solvent-free synthesis, controlled particle engineering)—can lead to dependency on potentially infringing technologies. A supplier’s process patent infringement could disrupt supply chains if litigation forces production halts.
Mitigating these pitfalls requires rigorous supplier qualification, clear contractual terms on quality and IP, and proactive compliance monitoring.

H2: Logistics & Compliance Guide for Calcium Stearate
Calcium stearate, a calcium salt of stearic acid, is widely used as a lubricant, release agent, stabilizer, and anti-caking agent in industries such as plastics, pharmaceuticals, food, and construction. Proper logistics handling and compliance with regulatory standards are essential to ensure safety, quality, and legal conformity during transportation, storage, and use.
1. Classification and Regulatory Status
- Chemical Name: Calcium Stearate
- CAS Number: 1529-23-0
- EC Number: 216-233-1
- Formula: Ca(C₁₈H₃₅O₂)₂
- Appearance: White, fine powder or granules, odorless
- Regulatory Classifications:
- GHS Classification: Not classified as hazardous under GHS (Globally Harmonized System) in most jurisdictions.
- REACH: Registered under EU REACH regulation (EC 1907/2006).
- FDA: Approved as an indirect food additive (21 CFR 178.375) and generally recognized as safe (GRAS) in food contact applications.
- USP/NF: Complies with United States Pharmacopeia standards when used in pharmaceuticals.
- Kosher/Halal: Available in certified grades for food and pharmaceutical use.
2. Packaging and Labeling
- Packaging Options:
- Multi-wall paper bags with polyethylene liner (net weight: 20–25 kg)
- Fiber drums (net weight: 200–250 kg)
- Bulk shipments in Intermediate Bulk Containers (IBCs) or big bags (500–1000 kg)
- Labeling Requirements:
- Product name: Calcium Stearate
- Batch number and manufacturing date
- Net weight
- Storage instructions (e.g., “Store in a cool, dry place”)
- Supplier name and contact information
- Regulatory statements (e.g., “Meets FDA 21 CFR for food contact”)
- GHS pictograms (if applicable), though typically not required
3. Storage Conditions
- Environment: Store in a cool, dry, well-ventilated area.
- Temperature: Ambient (15–25°C recommended); avoid prolonged exposure to temperatures above 60°C.
- Humidity: Low humidity to prevent clumping.
- Shelf Life: Typically 24–36 months when stored properly.
- Segregation: Keep away from strong oxidizing agents and acids.
4. Handling and Safety Precautions
- Personal Protective Equipment (PPE):
- Dust mask or respirator (for fine powders)
- Safety goggles
- Gloves (nitrile or similar)
- Protective clothing to avoid skin contact
- Dust Control:
- Handle in well-ventilated areas or use local exhaust ventilation.
- Avoid generating dust; use vacuum systems for cleanup.
- Fire Hazard: Non-flammable, but may emit toxic fumes (e.g., CO, CO₂) when exposed to high heat or fire.
- First Aid Measures:
- Inhalation: Move to fresh air; seek medical attention if irritation persists.
- Skin Contact: Wash with soap and water.
- Eye Contact: Rinse thoroughly with water for at least 15 minutes.
- Ingestion: Rinse mouth; do not induce vomiting. Seek medical advice if large amounts ingested.
5. Transportation Guidelines
- Transport Classification: Non-hazardous under IATA, IMDG, and ADR regulations.
- UN Number: Not regulated (UN 3077 may apply if impurities exceed thresholds, but typically not assigned).
- Packaging Group: Not applicable (non-hazardous).
- Documentation:
- Safety Data Sheet (SDS) required
- Commercial invoice
- Packing list
- Certificate of Analysis (CoA), especially for food/pharma grades
- Modes of Transport:
- Road, rail, air, and sea — all permitted without special restrictions.
- Ensure packages are sealed and protected from moisture.
6. Environmental and Disposal Considerations
- Environmental Impact: Low toxicity to aquatic life; biodegradable under aerobic conditions.
- Spill Management:
- Sweep or vacuum spilled material.
- Avoid dust generation.
- Collect in a sealed container for reuse or disposal.
- Waste Disposal:
- Dispose of in accordance with local, regional, and national regulations.
- May be disposed of in municipal or industrial landfills if permitted.
- Incineration in approved facilities is acceptable.
7. Regulatory Compliance Documentation
Suppliers and users should maintain:
– Safety Data Sheet (SDS): In accordance with GHS and local regulations (e.g., OSHA HazCom, CLP).
– Certificate of Analysis (CoA): Confirms purity, heavy metals, and compliance with food/pharma standards.
– Declaration of Compliance (DoC): For FDA, REACH, or other regulatory frameworks.
– Non-GMO, Kosher, Halal, or Organic Certifications (if applicable).
8. Special Considerations by Industry
- Pharmaceuticals: Must comply with current Good Manufacturing Practices (cGMP), USP-NF, and ICH guidelines.
- Food Industry: Confirm compliance with FDA 21 CFR, EFSA, and local food additive regulations.
- Plastics & Rubber: Typically used in non-regulated industrial applications; verify compatibility with processing temperatures.
- Construction: Used as a waterproofing agent; ensure compatibility with cementitious systems.
9. Import/Export Requirements
- Customs Tariff Code (HS Code): Typically 2915.70 (fatty acid salts and esters).
- Import Documentation:
- Bill of Lading/Air Waybill
- Commercial Invoice
- SDS and CoA
- Import license (if required by destination country)
- Export Controls: Generally not subject to export restrictions, but verify country-specific regulations.
10. Recommended Best Practices
- Conduct periodic audits of supplier compliance.
- Train personnel on safe handling and emergency procedures.
- Perform compatibility testing when introducing calcium stearate into new formulations.
- Maintain lot traceability for quality control and recall preparedness.
Conclusion
Calcium stearate is a low-hazard chemical with broad industrial applications. While it is not classified as dangerous for transport or storage, adherence to good manufacturing, handling, and documentation practices is crucial—especially in regulated sectors like food and pharmaceuticals. Maintaining up-to-date compliance documentation and following proper logistics protocols ensures safe and legal distribution worldwide.
Conclusion for Sourcing Calcium Stearate
In conclusion, sourcing calcium stearate requires careful consideration of several key factors to ensure quality, cost-effectiveness, and reliability. It is essential to identify suppliers with strong manufacturing standards, consistent product quality, and compliance with relevant industry regulations (such as food-grade, pharmaceutical, or industrial specifications). Evaluating factors such as purity, particle size, origin of raw materials (animal or vegetable-based), and environmental or sustainability practices can significantly impact suitability for the intended application.
Additionally, establishing long-term relationships with reputable suppliers, conducting regular audits, and performing batch testing can help maintain supply chain integrity. Global sourcing options should be balanced with logistical considerations, lead times, and regulatory requirements in the destination market.
Ultimately, a strategic approach to sourcing calcium stearate—focusing on quality assurance, supplier reliability, and cost efficiency—will support consistent production, product performance, and compliance across various industries including plastics, cosmetics, pharmaceuticals, and food processing.