The global blood transfusion bags market is experiencing steady growth, driven by rising demand for safe blood collection, storage, and transfusion solutions amid increasing surgical procedures, trauma cases, and blood disorders. According to a report by Mordor Intelligence, the blood transfusion bags market was valued at approximately USD 2.1 billion in 2023 and is projected to grow at a compound annual growth rate (CAGR) of over 6.2% during the forecast period from 2024 to 2029. This expansion is fueled by advancements in blood bag technology—including the development of additive solutions that extend shelf life, integrated sampling systems, and improved biocompatibility—as well as heightened focus on infection control and regulatory compliance across healthcare systems. In this competitive landscape, a select group of manufacturers have emerged as industry leaders, combining innovation, global reach, and rigorous quality standards to meet the evolving needs of blood banks, hospitals, and transfusion centers worldwide. Below is a data-informed overview of the top 9 blood transfusion bag manufacturers shaping the future of transfusion medicine.
Top 9 Blood Transfusion Bag Manufacturers 2026
(Ranked by Factory Capability & Trust Score)
#1 Tianhe Pharmaceutical Co., Ltd.
Domain Est. 2007
Website: jxtianhe.com
Key Highlights: No.1 blood bag manufacturer in China. Tianhe’s goal is focusing on research, development and manufacture of disposable plastic systems for blood transfusion….
#2 Haemonetics®
Domain Est. 1996
Website: haemonetics.com
Key Highlights: Haemonetics provides a suite of innovative medical technology solutions that improve the quality, effectiveness and efficiency of care….
#3 Macopharma
Domain Est. 1998
Website: macopharma.com
Key Highlights: Macopharma offers a range of blood processing solutions combining expertise on disposables, equipments, softwares and processing guidelines….
#4 JMS Blood Bags
Domain Est. 1999
Website: jmsna.net
Key Highlights: Blood bags manufactured by JMS have a long reputation for reliability and superior product quality. Our manufacturing facility meets ISO13485 and other Quality ……
#5 BLOOD BAGS
Domain Est. 2000
Website: demophorius.com
Key Highlights: Demotek high quality blood bags are suitable for the collection of blood and blood components and for the filtration of leukocytes from whole blood….
#6 Terumopenpol
Domain Est. 2002
Website: terumopenpol.com
Key Highlights: Practicing the concept of ” Quality First ” approach in manufacturing, Terumo Penpol has been serving Blood Centers across the globe for more than thirty years….
#7 Blood Bags
Domain Est. 2005
Website: genesisbps.com
Key Highlights: Blood bags, transfer-packs for distribution in USA only. Genesis BPS offers a full range of blood bags, transfer bags, iv sets and transfer sets….
#8 Terumo Blood Bags
Domain Est. 2011
Website: terumobct.com
Key Highlights: Terumo Blood Bags. Optimize whole blood collection, component processing, and storage with our comprehensive range of blood bag systems….
#9 Bionic Medical Products
Founded: 1969
Website: bionic-jms.de
Key Highlights: JMS Blood Bag Systems. Expertise, Quality, and Reliable Service for European Transfusion Medicine. Since 1969, JMS has been manufacturing blood bags and has ……
Expert Sourcing Insights for Blood Transfusion Bag

H2: 2026 Market Trends for Blood Transfusion Bags
The global market for blood transfusion bags is expected to experience significant transformation by 2026, driven by advancements in medical technology, increasing demand for safe blood handling, and evolving healthcare infrastructure. Several key trends are shaping the trajectory of this market:
-
Growing Demand for Single-Use and Integrated Systems
By 2026, there is a clear shift toward single-use, sterile blood transfusion bags with integrated features such as pre-attached tubing, anticoagulant coatings, and closed-system designs. These innovations reduce contamination risks and streamline transfusion procedures, particularly in emergency and surgical settings. -
Adoption of Advanced Materials
Manufacturers are increasingly using medical-grade polyvinyl chloride (PVC) and non-PVC alternatives like TPU (thermoplastic polyurethane) and multilayer films to enhance biocompatibility, flexibility, and shelf life. Non-PVC bags are gaining traction due to growing environmental and health concerns over phthalate plasticizers. -
Rising Blood Donation and Transfusion Rates
Expanding blood donation programs, especially in emerging economies in Asia-Pacific and Africa, are boosting the consumption of transfusion bags. Governments and NGOs are investing in blood bank infrastructure, directly increasing product demand. -
Technological Integration and Smart Bags
The integration of RFID tags, QR codes, and temperature-sensitive indicators into transfusion bags is expected to grow. These “smart” features improve traceability, reduce transfusion errors, and ensure better cold chain management—critical for maintaining blood integrity. -
Stringent Regulatory Standards
Regulatory bodies such as the FDA and EMA are enforcing stricter guidelines on sterility, labeling, and shelf-life validation. Compliance with ISO 1135 and other international standards is becoming a prerequisite for market entry, pushing manufacturers toward innovation and quality assurance. -
Impact of Pandemics and Disaster Preparedness
Lessons from recent global health crises have increased focus on emergency blood supply readiness. By 2026, national stockpiling initiatives and mobile blood collection units are expected to drive demand for robust, transport-friendly transfusion bag systems. -
Market Consolidation and Strategic Collaborations
Leading players such as Terumo BCT, Fresenius Kabi, MacoPharma, and Haemonetics are engaging in mergers, R&D partnerships, and geographic expansions to strengthen their market position. This consolidation is expected to accelerate innovation and improve supply chain resilience. -
Sustainability and Eco-Friendly Packaging
Environmental concerns are pushing companies to develop recyclable materials and reduce plastic waste. By 2026, eco-conscious procurement policies in healthcare institutions will likely favor suppliers offering sustainable transfusion solutions.
In conclusion, the 2026 blood transfusion bag market will be characterized by technological sophistication, regulatory rigor, and a strong emphasis on safety and sustainability. Stakeholders who invest in innovation, compliance, and scalable manufacturing are poised to lead this evolving landscape.

Common Pitfalls Sourcing Blood Transfusion Bags: Quality and Intellectual Property Risks
Sourcing blood transfusion bags involves critical considerations beyond cost and availability. Overlooking quality assurance and intellectual property (IP) rights can lead to regulatory non-compliance, patient safety risks, and legal liabilities. Below are key pitfalls to avoid:
Quality-Related Pitfalls
Inadequate Regulatory Compliance
Suppliers may claim compliance with international standards (e.g., ISO 13485, FDA 21 CFR Part 820, or EU MDR), but documentation can be outdated or falsified. Failure to verify current certifications and conduct on-site audits increases the risk of sourcing non-compliant products, potentially resulting in shipment rejections or market withdrawals.
Substandard Material Composition
The plasticizers (e.g., DEHP, TOTM) and polymer materials (e.g., PVC, polyolefins) used in transfusion bags affect blood component stability and patient safety. Sourcing from manufacturers using inferior or untested materials can lead to leachables and extractables, compromising blood quality and posing health risks.
Inconsistent Manufacturing Processes
Lack of process validation and quality control can result in defects such as leaks, poor weld integrity, or inconsistent anticoagulant distribution. These flaws may not be apparent during initial inspections but can cause failures during clinical use.
Insufficient Validation of Biocompatibility and Sterility
Blood bags must be sterile and biocompatible. Relying solely on supplier-provided test reports without independent verification or batch-specific certificates of analysis (CoA) exposes buyers to risks of endotoxin contamination or adverse patient reactions.
Poor Supply Chain Traceability
Inadequate lot tracking and lack of serialization hinder effective recalls in case of defects. Suppliers with weak documentation practices make it difficult to trace materials back to origin, increasing patient safety and regulatory risks.
Intellectual Property-Related Pitfalls
Infringement of Patented Designs or Technologies
Many blood bag systems incorporate patented features such as integrated sampling ports, specialized tubing connectors, or anticoagulant formulations. Sourcing from suppliers using third-party patented technology without licensing exposes the buyer to infringement lawsuits, even if unintentional.
Counterfeit or Copycat Products
Low-cost suppliers may offer products that mimic branded designs but lack proper IP clearance. These counterfeits often compromise on quality and may infringe on design patents or trade dress, leading to legal action and reputational damage.
Unclear Ownership of Customized Designs
When working with suppliers to develop custom blood bag configurations, failure to define IP ownership in contracts may result in disputes. Suppliers could claim rights to modifications or prevent future sourcing from alternative vendors.
Lack of Freedom-to-Operate (FTO) Analysis
Buyers often overlook conducting or requiring an FTO analysis before procurement. Without this, there’s a risk of introducing products into markets where key patents are enforced, potentially leading to injunctions or costly litigation.
Mitigation Strategies
- Conduct thorough due diligence, including site audits and review of regulatory documentation.
- Require material certifications, biocompatibility reports, and sterilization validation data.
- Perform independent testing on initial and periodic batches.
- Engage legal counsel to assess IP risks and ensure supplier warranties on non-infringement.
- Include clear IP ownership clauses in sourcing agreements.
- Partner with reputable, certified manufacturers with transparent supply chains.
Avoiding these pitfalls ensures not only regulatory and clinical compliance but also protects patient safety and organizational integrity.
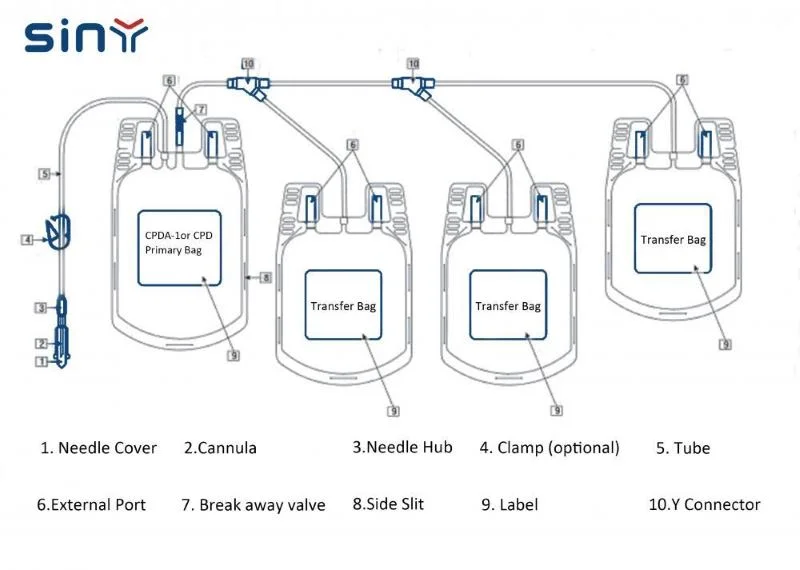
Logistics & Compliance Guide for Blood Transfusion Bags
Overview and Importance
Blood transfusion bags are critical medical devices used to collect, store, transport, and transfuse blood and its components. Ensuring proper logistics and compliance throughout the supply chain is essential to maintain blood safety, efficacy, and patient well-being. This guide outlines key considerations for handling, transporting, storing, and complying with regulatory standards for blood transfusion bags.
Regulatory Framework
Blood transfusion bags must comply with stringent international, national, and regional regulations. Key regulatory bodies include:
– FDA (U.S. Food and Drug Administration) – Regulates blood collection bags under 21 CFR Part 606 and 640.
– European Medicines Agency (EMA) and Directive 2002/98/EC – Governs blood and blood components in the EU.
– World Health Organization (WHO) – Provides global guidelines on blood safety and transfusion practices.
– AABB (formerly American Association of Blood Banks) – Offers accreditation standards and best practices.
Manufacturers and transfusion services must ensure compliance with Good Manufacturing Practices (GMP), Good Distribution Practices (GDP), and traceability requirements.
Temperature Control and Cold Chain Management
Maintaining the correct temperature is vital for preserving blood components:
– Whole Blood and Red Blood Cells (RBCs): 1–6°C (34–43°F)
– Platelets: 20–24°C with continuous agitation
– Fresh Frozen Plasma (FFP): ≤ -18°C (≤ 0°F), typically stored at -30°C
– Cryoprecipitate: ≤ -18°C after thawing and pooling
Transportation must use validated refrigerated containers with temperature monitoring (data loggers). Any temperature excursion must be documented and assessed for impact on blood safety.
Packaging and Labeling Requirements
Proper packaging and labeling ensure traceability and safety:
– Each transfusion bag must have a unique identifier (e.g., ISBT 128 barcode).
– Labels must include: donor ID, blood group (ABO/Rh), component type, collection date, expiration date, storage conditions, and any additives.
– Packaging must be leak-proof, sterile, and protect against physical damage and contamination.
– Use of tamper-evident seals is required.
Storage Conditions
- Blood products should be stored in dedicated, monitored refrigerators or freezers with alarms.
- Avoid placement near freezer walls or cooling elements to prevent freezing of RBCs.
- Separate storage for different blood types and components is recommended to prevent errors.
- Regular maintenance and temperature validation of storage units are mandatory.
Transportation and Distribution
- Use only authorized and trained personnel for transport.
- Vehicles or containers must be validated for temperature maintenance and equipped with real-time monitoring.
- Minimize transit time; emergency transports should have contingency plans.
- Documentation must accompany each shipment, including chain-of-custody records.
Traceability and Documentation
Full traceability from donor to recipient is required:
– Maintain electronic records of collection, testing, processing, storage, distribution, and transfusion.
– Implement systems for rapid recall in case of contamination or adverse events.
– Adhere to ISBT 128 standards for coding and data management.
Safety and Quality Assurance
- Regular audits and quality control checks of logistics processes.
- Staff training on blood handling, cold chain management, and emergency procedures.
- Validation of all equipment (refrigerators, freezers, transport containers).
- Reporting and investigation of deviations (e.g., temperature breaches, labeling errors).
Disposal and Waste Management
- Expired or contaminated blood products must be disposed of according to biohazard regulations.
- Use approved medical waste disposal services.
- Maintain records of disposal for compliance and audit purposes.
Emergency Preparedness
- Establish protocols for power outages, equipment failure, and natural disasters.
- Maintain backup refrigeration and emergency transport options.
- Coordinate with regional blood banks for mutual aid during shortages.
Conclusion
Proper logistics and compliance for blood transfusion bags are essential to ensure patient safety and regulatory adherence. By following standardized procedures for storage, transport, labeling, and documentation, healthcare providers and blood banks can maintain the integrity and efficacy of blood products throughout the supply chain. Regular training, audits, and continuous improvement are key to sustaining high-quality blood transfusion services.
Conclusion for Sourcing Blood Transfusion Bags:
Sourcing blood transfusion bags is a critical process that directly impacts patient safety, treatment efficacy, and the overall efficiency of healthcare delivery. A successful sourcing strategy requires careful evaluation of suppliers based on quality certifications (such as ISO and FDA compliance), material safety, sterility standards, regulatory adherence, and supply chain reliability. It is essential to select blood transfusion bags made from medical-grade materials (e.g., DEHP-free or alternative plasticizers) that ensure blood component compatibility and reduce health risks. Additionally, cost-effectiveness, scalability, and timely delivery must be balanced with uncompromising quality standards. Establishing long-term partnerships with reputable manufacturers, conducting regular audits, and staying updated on technological advancements and regulatory changes will enhance sourcing effectiveness. Ultimately, a well-structured sourcing approach ensures the availability of safe, reliable, and high-quality blood transfusion bags, supporting optimal patient outcomes and strengthening healthcare system resilience.