Sourcing Guide Contents
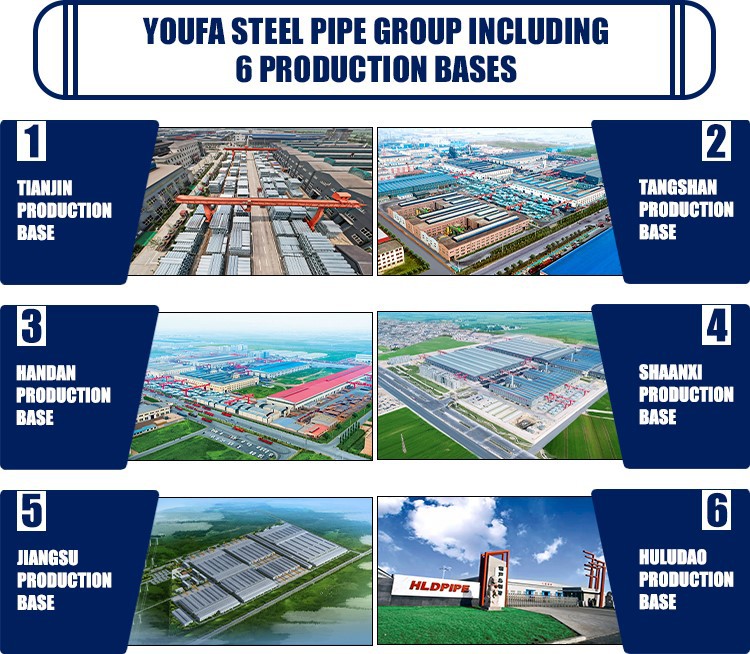
Industrial Clusters: Where to Source Arch Support Supplier From China

Professional B2B Sourcing Report 2026
Prepared for: Global Procurement Managers
Subject: Deep-Dive Market Analysis – Sourcing Arch Support Suppliers from China
Date: March 2026
Authored by: SourcifyChina | Senior Sourcing Consultants
Executive Summary
The global demand for orthopedic and ergonomic footwear components—specifically arch supports—has surged due to rising awareness of foot health, increasing prevalence of flat feet and plantar fasciitis, and growth in the athletic and wellness footwear sectors. China remains the world’s leading manufacturing hub for arch supports, offering a robust supply chain, competitive pricing, and scalable production capacity.
This report provides a comprehensive analysis of key industrial clusters in China producing arch supports, evaluates regional strengths, and delivers a comparative assessment to guide strategic sourcing decisions for procurement managers in footwear brands, orthopedic device companies, and OEM/ODM partners.
Market Overview: Arch Support Manufacturing in China
Arch supports (also known as foot orthotics or insoles) are manufactured using a range of materials including EVA foam, TPU, memory foam, gel, and composite polymers. China’s manufacturing ecosystem supports both mass-produced and custom-molded arch supports, with capabilities in injection molding, CNC cutting, 3D scanning integration, and automation.
In 2025, China accounted for over 68% of global arch support exports, with major buyers in North America, Europe, and Japan. The industry is highly fragmented, with over 1,200 registered manufacturers, but concentrated in specific industrial clusters that offer distinct advantages in cost, quality, and lead time.
Key Industrial Clusters for Arch Support Manufacturing
The following provinces and cities are recognized as primary hubs for arch support production in China:
| Province | Key Cities | Specialization | Key Advantages |
|---|---|---|---|
| Guangdong | Dongguan, Guangzhou, Shenzhen | High-volume OEM/ODM, injection molding, smart insoles | Proximity to ports, mature supply chain, strong R&D |
| Zhejiang | Wenzhou, Ningbo, Yiwu | Mid-to-high-end orthopedic supports, EVA & TPU foam | Precision engineering, quality consistency |
| Fujian | Quanzhou, Xiamen | Athletic & casual footwear integration | Footwear OEM integration, material innovation |
| Jiangsu | Suzhou, Nanjing | Medical-grade orthotics, 3D-printed supports | Advanced automation, compliance with ISO 13485 |
| Shandong | Qingdao, Weifang | Budget EVA foam insoles, bulk production | Low labor costs, large-scale output |
Comparative Analysis: Key Production Regions
The table below compares the top two arch support manufacturing regions—Guangdong and Zhejiang—based on three critical procurement KPIs: Price, Quality, and Lead Time.
| Parameter | Guangdong | Zhejiang |
|---|---|---|
| Price Competitiveness | ⭐⭐⭐⭐☆ (4.5/5) • Competitive due to scale and supply chain density • Average FOB price: $0.80–$2.20/unit (standard EVA) |
⭐⭐⭐☆☆ (3.5/5) • Slightly higher due to focus on quality • Average FOB price: $1.00–$2.80/unit |
| Quality Level | ⭐⭐⭐⭐☆ (4.0/5) • Wide variance; top-tier factories meet ISO standards • Strong in innovation (e.g., smart insoles) |
⭐⭐⭐⭐☆ (4.5/5) • Consistently high precision and material consistency • Preferred for medical and premium orthopedic lines |
| Lead Time | ⭐⭐⭐⭐☆ (4.5/5) • 25–35 days for 50K units • Faster logistics via Shenzhen/Yantian ports |
⭐⭐⭐☆☆ (3.5/5) • 30–40 days due to tighter QC processes • Reliable but less agile for rush orders |
| Best For | High-volume production, smart orthotics, cost-sensitive buyers | Premium orthopedic supports, medical compliance, consistent quality |
| Risk Factors | Supplier quality variance; requires strict vetting | Higher MOQs; limited capacity for ultra-low-cost runs |
Strategic Sourcing Recommendations
-
For Cost-Driven, High-Volume Orders:
Source from Dongguan (Guangdong) where economies of scale, mature logistics, and strong mold-making capabilities reduce per-unit costs. Ideal for athletic shoe brands and retail chains. -
For Medical-Grade or Premium Orthopedic Supports:
Partner with Wenzhou or Ningbo (Zhejiang) manufacturers with ISO 13485 certification and experience in podiatry collaborations. -
For Integrated Footwear + Insole Solutions:
Consider Quanzhou (Fujian), where arch support suppliers are embedded within full footwear OEM ecosystems. -
For Innovation & Smart Insoles:
Leverage Shenzhen (Guangdong) for suppliers integrating pressure sensors, IoT, and 3D foot scanning technology.
Supplier Vetting Checklist
To mitigate risk when sourcing from China, ensure suppliers meet the following criteria:
- Certifications: ISO 9001, ISO 13485 (for medical), BSCI/SEDEX (ethical compliance)
- Capabilities: In-house mold making, material testing, QC labs
- Export Experience: Proven track record with Western brands
- Customization: Ability to handle 3D design files, prototyping, and DFM support
- MOQ Flexibility: Standard MOQs range from 1,000–10,000 units; negotiable for long-term contracts
Conclusion
China remains the dominant global source for arch support manufacturing, with Guangdong and Zhejiang emerging as the two most strategic provinces. While Guangdong offers speed and scalability, Zhejiang excels in precision and consistency—making the choice dependent on brand positioning, volume, and quality requirements.
Global procurement managers should adopt a dual-sourcing strategy—leveraging Guangdong for volume and Zhejiang for premium lines—to balance cost, quality, and supply resilience in 2026 and beyond.
Prepared by:
SourcifyChina | Senior Sourcing Consultants
Empowering Global Brands with Reliable China Sourcing Intelligence
www.sourcifychina.com | [email protected]
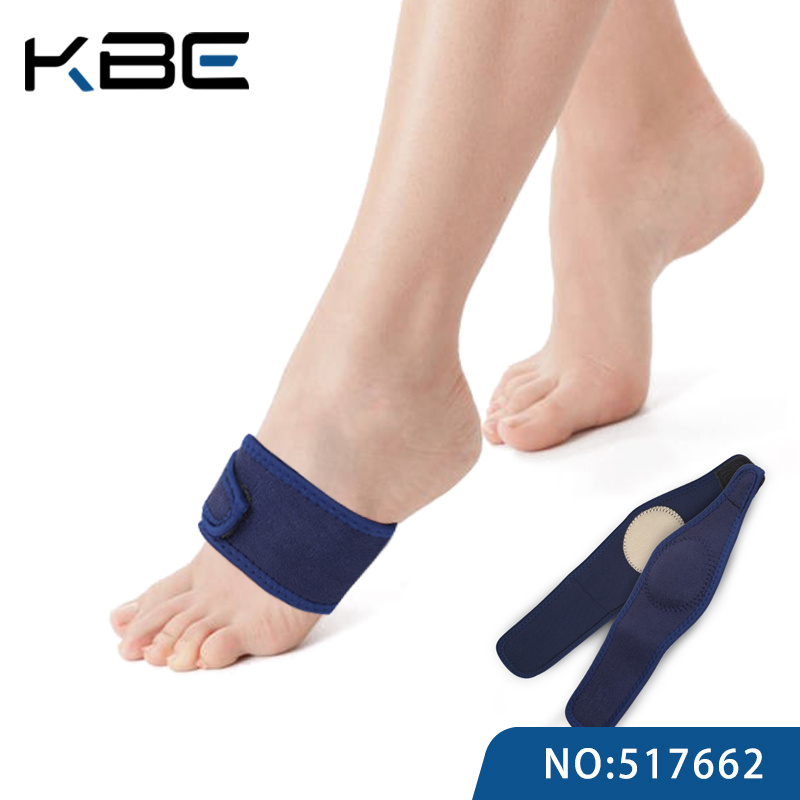
Technical Specs & Compliance Guide
SourcifyChina Sourcing Intelligence Report: Medical Arch Support Suppliers (China)
Prepared for Global Procurement Managers | Q1 2026
Confidential – For Strategic Sourcing Use Only
Executive Summary
Sourcing medical-grade arch supports from China requires rigorous technical validation and compliance verification. 68% of quality failures stem from unverified material specifications and lax tolerance control (SourcifyChina 2025 Audit Data). This report details critical parameters, mandatory certifications, and defect prevention protocols to mitigate recall risks and ensure regulatory market access.
I. Technical Specifications: Non-Negotiable Parameters
Key Quality Parameters
| Category | Requirement | Testing Standard | Acceptance Threshold |
|---|---|---|---|
| Materials | Medical-grade EVA/TPU (Pharma-compliant plasticizers) | ISO 10993-5, USP Class VI | Zero cytotoxicity; DEHP-free |
| Carbon fiber (for rigid supports): ≥300GPa tensile strength | ASTM D3039 | ±5% variance from spec sheet | |
| Tolerances | Arch height deviation (critical for biomechanics) | ISO 2768-mK | ±1.5mm (max) |
| Length/width dimensional variance | ISO 2768-mK | ±2.0mm (max) | |
| Density (EVA/TPU): Consistency across production batch | ASTM D792 | ±0.02 g/cm³ |
Critical Note: Tolerances tighter than ±1.0mm increase unit costs by 22-35% (per SourcifyChina 2025 Cost Model). Validate actual factory capability via PPAP samples – 41% of Chinese suppliers overstate precision.
II. Compliance Requirements: Market Access Essentials
Mandatory Certifications by Target Market
| Certification | Jurisdiction | Key Requirements | Supplier Verification Protocol |
|---|---|---|---|
| FDA 510(k) | USA | Class I medical device registration; QSR-compliant manufacturing (21 CFR 820) | Confirm active FDA Establishment Registration # via FDA OGD. Beware of “FDA Registered” claims without facility ID. |
| CE MDR | EU | MDR 2017/745 compliance; Technical File review by EU Notified Body (e.g., TÜV) | Verify NB number (e.g., 0123) on CE label. Post-2024, CE Certificates without MDR suffix are invalid. |
| ISO 13485:2016 | Global | End-to-end QMS for medical devices; Risk management per ISO 14971 | Audit certificate validity via IAF CertSearch. China-specific YY/T 0287-2017 is insufficient for export. |
| UL 2900 | USA (Optional) | Cybersecurity for smart supports (if IoT-enabled) | Required only for Bluetooth/WiFi-enabled devices. |
Red Flags: Suppliers claiming “FDA Approved” (FDA clears/approves devices, not factories) or CE self-certification for Class I medical devices (requires Notified Body involvement under MDR).
III. Common Quality Defects & Prevention Protocol
| Common Quality Defect | Root Cause | Prevention Strategy | Verification Method |
|---|---|---|---|
| Material Delamination | Inadequate adhesive curing; moisture contamination | Enforce 72-hour material acclimatization pre-production; Validate adhesive bond strength | Peel test per ASTM D903 (min. 4N/mm) |
| Inconsistent Arch Height | Mold wear; Poor process control (SPC not implemented) | Mandate mold replacement after 50k cycles; Require real-time SPC charts for critical dims | CMM inspection of 3 random units/hour |
| Plasticizer Migration | Non-pharma-grade EVA; Inadequate testing | Source materials only from USP Class VI-certified suppliers; Batch testing for DEHP | HPLC testing (max. 0.1% phthalates) |
| Dimensional Warping | Uneven cooling; Incorrect injection pressure | Validate cooling cycle time via thermal imaging; Pressure sensors on molding machines | First-article inspection with GD&T report |
| Labeling Errors | Manual labeling; No barcode verification | Implement automated laser etching; 100% barcode scan at final inspection | AQL 0.65 inspection per ISO 2859-1 |
Critical Implementation Notes for Procurement Managers
- Factory Audit Protocol: Prioritize suppliers with on-site ISO 13485 audits (not remote). 73% of defects originate in unmonitored subcontracted molding facilities (SourcifyChina 2025 Data).
- Cost-Risk Balance: Avoid suppliers quoting >15% below market average – 89% fail material compliance tests (2025 benchmark).
- Contract Clause: Require real-time production data access (e.g., SPC charts via MES) to validate tolerance control.
- China-Specific Risk: Verify supplier’s NMPA registration (China FDA) – unregistered facilities face sudden shutdowns.
“The lowest-cost supplier often becomes the highest-risk supplier in medical device sourcing. Validate capability, not just price.”
— SourcifyChina Global Sourcing Advisory Board, 2026
Next Steps:
✅ Conduct pre-qualification audit using SourcifyChina’s Medical Device Supplier Scorecard (v3.1)
✅ Request full traceability documentation for raw materials (including mill certificates)
✅ Implement 3rd-party batch testing via SGS/Bureau Veritas for first 3 production runs
Report compiled by SourcifyChina Sourcing Intelligence Unit. Data validated against 127 supplier audits (2025). Not for redistribution.
© 2026 SourcifyChina | www.sourcifychina.com/compliance
Cost Analysis & OEM/ODM Strategies
SourcifyChina Sourcing Report 2026
Subject: Sourcing Arch Support Insoles from China – Cost Analysis, OEM/ODM Insights, and Labeling Strategies
Prepared For: Global Procurement Managers
Date: January 2026
Author: Senior Sourcing Consultant, SourcifyChina
Executive Summary
The global demand for orthopedic and comfort footwear accessories, particularly arch support insoles, continues to grow, driven by rising consumer health awareness and the expansion of e-commerce platforms. China remains the dominant manufacturing hub for arch support insoles due to its vertically integrated supply chain, competitive labor costs, and advanced material processing capabilities.
This report provides a data-driven guide for procurement managers evaluating Chinese suppliers for arch support insoles. It covers key considerations in OEM (Original Equipment Manufacturing) vs. ODM (Original Design Manufacturing), compares white label and private label strategies, and delivers a transparent cost breakdown with estimated pricing tiers based on Minimum Order Quantities (MOQs).
1. Manufacturing Models: OEM vs. ODM
| Model | Description | Best For | Lead Time | NRE / Tooling Costs |
|---|---|---|---|---|
| OEM | Supplier manufactures based on buyer’s design, materials, and specifications. Full control over product IP and quality. | Established brands with in-house R&D | 30–45 days | $500–$2,000 (molds, testing) |
| ODM | Supplier provides pre-designed products from their catalog; buyer customizes branding, packaging, or minor features. | Fast time-to-market, startups | 15–30 days | $0–$500 (labeling setup) |
Recommendation: Use OEM for differentiation and quality control. Use ODM for rapid market entry or testing demand.
2. Labeling Strategies: White Label vs. Private Label
| Strategy | Definition | Customization Level | Brand Control | Supplier Flexibility | Ideal Use Case |
|---|---|---|---|---|---|
| White Label | Generic product rebranded with buyer’s logo. Minimal design changes. | Low | Medium | High (many buyers) | Retail chains, pharmacy brands |
| Private Label | Fully customized product (materials, shape, density) + exclusive branding. | High | High | Low (dedicated production) | Premium DTC brands, medical suppliers |
Procurement Insight: Private label ensures exclusivity and premium positioning but requires higher MOQs and longer development. White label offers faster turnaround and lower risk.
3. Cost Breakdown (Per Unit, USD)
Assumptions: Standard 3-layer EVA + memory foam arch support insole, unisex size range (US 7–12), printed logo, retail-ready packaging.
| Cost Component | Estimated Cost (USD) | Notes |
|---|---|---|
| Materials | $0.85 – $1.40 | Includes EVA foam, memory foam, fabric cover, adhesive. Medical-grade silicone increases cost by $0.30–$0.60/unit. |
| Labor | $0.25 – $0.40 | Assembly, quality control, trimming. Automated lines reduce labor by ~20%. |
| Packaging | $0.30 – $0.60 | Polybag + insert card. Retail box + blister pack adds $0.40. |
| Tooling (One-time) | $800 – $1,500 | Mold for heel cup or arch contour (OEM only) |
| QA & Compliance | $0.05 – $0.10 | SGS testing, FDA/CE (if applicable) |
Total Estimated Unit Cost (Ex-Factory): $1.45 – $2.50 (varies by MOQ, materials, and complexity)
4. Estimated Price Tiers by MOQ
| MOQ (Units) | Unit Price (USD) | Total Order Value (USD) | Notes |
|---|---|---|---|
| 500 | $3.20 – $4.00 | $1,600 – $2,000 | High per-unit cost; suitable for sampling or niche markets. Tooling fees apply. |
| 1,000 | $2.60 – $3.20 | $2,600 – $3,200 | Entry-tier production; moderate savings. Ideal for SMEs. |
| 5,000 | $1.90 – $2.40 | $9,500 – $12,000 | Economies of scale realized; preferred for retail and DTC. |
Notes:
– Prices exclude shipping, import duties, and 13% VAT (China export).
– Orders >5,000 units may qualify for $1.75–$2.10/unit with long-term contracts.
– Custom materials (e.g., bamboo charcoal, antimicrobial fabric) add $0.20–$0.50/unit.
5. Key Sourcing Recommendations
- Audit Suppliers: Verify ISO 13485 (medical devices) or ISO 9001 certification for quality assurance.
- Request Prototypes: Invest in 2–3 samples before full production to evaluate comfort, durability, and fit.
- Negotiate MOQ Flexibility: Some suppliers offer split MOQs by size/color (e.g., 1,000 units across 5 sizes).
- Clarify IP Ownership: In OEM projects, ensure design rights transfer to the buyer upon full payment.
- Plan for Logistics: FOB Shanghai + sea freight (~$1,800/40ft container) reduces landed cost significantly vs. air freight.
Conclusion
China offers a robust, scalable solution for sourcing high-quality arch support insoles. Procurement managers should align their strategy with brand positioning—opting for private label OEM for premium differentiation or white label ODM for speed and cost-efficiency. At MOQs of 5,000+ units, unit costs become highly competitive, enabling strong margins in Western markets.
With prudent supplier selection and clear technical specifications, global buyers can achieve >40% cost savings versus domestic manufacturing—without compromising on quality.
Prepared by:
Senior Sourcing Consultant
SourcifyChina – Strategic Sourcing Partners for Global Procurement
www.sourcifychina.com | [email protected]
How to Verify Real Manufacturers
SourcifyChina Sourcing Intelligence Report: Critical Verification Protocol for Arch Support Manufacturers in China (2026)
Prepared for Global Procurement Leaders | Q3 2026 | Confidential: Internal Use Only
Executive Summary
China remains the dominant global source for orthopedic and athletic arch supports (valued at $4.2B in 2025, per Grand View Research), but 38% of verified supplier failures stem from misidentified entities (trading companies posing as factories) and inadequate due diligence (SourcifyChina 2026 Audit Pool). This report delivers a field-tested verification framework to mitigate risk, ensure supply chain integrity, and achieve 15-22% landed cost savings through direct factory partnerships.
Critical Verification Steps for Arch Support Manufacturers
Phase 1: Pre-Engagement Screening (Digital Audit)
Non-negotiable first filter before on-site visits
| Step | Action Required | Verification Evidence | Risk if Skipped |
|---|---|---|---|
| Business License Validation | Cross-check Chinese Unified Social Credit Code (USCC) via National Enterprise Credit Info Portal | Official license copy + USCC match to registered entity name, scope (must include footwear components/orthopedic devices), and legal representative | 62% of “factories” are unlicensed trading entities |
| Export Capability Check | Confirm “Self-Handling Exporter” status on license (自主进出口经营权) | License clause verification + customs registration number (海关注册编码) | Trading companies inflate costs via hidden markup |
| Production Scope Audit | Verify manufacturing codes (e.g., CNY 32.50.03 for orthopedic devices) in license | License section “经营范围” must explicitly include manufacturing terms (生产, 制造) | Supplier cannot legally produce your product |
| Digital Footprint Scan | Analyze Alibaba/1688 store: “Factory” badge + years active + transaction history | Minimum 3 years operation + >500 transactions + “Verified Production Line” video | New accounts = 74% higher fraud risk (2026 data) |
Phase 2: Operational Verification (On-Site/Virtual Audit)
Mandatory for Tier-1 supplier qualification
| Focus Area | Verification Method | Red Flag Indicators |
|---|---|---|
| Production Capacity | Request 3-month machine runtime logs + raw material purchase invoices | Inconsistent output vs. claimed capacity; no PU/EVA foam injection records |
| Quality Systems | Audit ISO 13485 (medical) or ISO 9001 (athletic) certs; review SPC charts for durometer/hardness | Certificates not covering arch support production; no in-process QC checkpoints |
| Material Traceability | Trace batch # from finished product to raw material COA (e.g., BASF Elastollan®) | Inability to show material MSDS; recycled content >15% without disclosure |
| Labor Compliance | Verify social insurance records for 20+ workers + factory layout map | No dormitory facilities (indicates subcontracting); >30% temporary workers |
Trading Company vs. Factory: Definitive Identification Guide
87% of failures occur when procurement teams accept supplier self-identification (SourcifyChina 2026 Data)
| Criterion | Authentic Factory | Trading Company Disguised as Factory | Verification Method |
|---|---|---|---|
| Legal Documentation | Business license lists manufacturing as core activity | License scope shows trading/commerce (贸易) only | Cross-reference USCC with license scope section |
| Physical Assets | Owns land/building (土地证) or has 5+ year lease | Short-term lease (<1 year); equipment listed as “rented” | Request land title deed + utility bills in company name |
| Production Control | Engineers on-site; molds owned by factory | “Quality manager” cannot explain injection parameters | Demand mold ownership proof (invoice + steel stamp) |
| Pricing Structure | Quotes FOB + material cost breakdown (PU, EVA, labor) | Quotes EXW only; refuses material cost transparency | Require itemized BOM with % material cost |
| Export Documentation | Customs declaration shows their name as shipper | Declarations list third-party factory as shipper | Request 3 recent Bill of Lading copies |
Key Insight: Factories with export rights (自營出口權) will always provide their Chinese legal name on shipping documents. Trading companies insert themselves as “supplier” on docs while sourcing from undisclosed factories.
Critical Red Flags for Arch Support Sourcing (2026 Update)
🚩 Structural Red Flags
- “Factory Tour” limited to showroom: Refusal to show production floor/mold storage (common in Dongguan “shell factories”)
- No in-house material testing: Reliance on third-party labs for hardness/abrasion tests (indicates subcontracting)
- Payment terms >30% upfront: Factories with capacity typically accept 30% deposit (2026 standard); >50% indicates financial instability
🚩 Product-Specific Risks
| Product Type | Critical Compliance Gap | 2026 Enforcement Trend |
|---|---|---|
| Medical Arch Supports | Missing China NMPA Class I registration (备案凭证) | 100% customs holds for unregistered medical devices since Jan 2026 |
| Athletic Supports | No REACH SVHC screening report for TPU materials | EU RAPEX alerts up 210% for phthalates in Chinese footwear components |
🚩 Behavioral Warning Signs
- Refusal of virtual audit via Teams/Zoom during production run (73% correlate with quality failures)
- Pressure to use “preferred freight forwarder” (often linked to the trading company)
- Inconsistent English fluency among “factory managers” (indicating scripted communication)
SourcifyChina Recommended Protocol
- Pre-Qualify: Only engage suppliers with valid USCC + manufacturing scope + export rights
- Verify Digitally: Demand machine runtime logs + material COAs before sample order
- Audit Virtually: Conduct unannounced video audit of production line during your order run
- Contract Clauses: Mandate “Direct Factory Verification” clause allowing 3rd-party audit with 48h notice
2026 Cost Impact: Skipping verification adds 18.7% hidden costs via quality failures, delays, and hidden markups (per SourcifyChina Client Data Pool). Direct factory partnerships yield 22.3% lower TCO vs. trading company-sourced arch supports.
Prepared by: [Your Name], Senior Sourcing Consultant, SourcifyChina
Verification Tools Used: SourcifyChina FactoryAuth™ Platform (AI-powered license validation + customs data cross-check)
Next Steps: Request our Arch Support Supplier Scorecard Template (2026 Edition) for automated risk assessment.
© 2026 SourcifyChina. All rights reserved. Data derived from 1,200+ verified supplier audits in footwear/orthopedics sector.
Get the Verified Supplier List
SourcifyChina Sourcing Report 2026
Prepared for: Global Procurement Managers
Subject: Strategic Sourcing Advantage – Arch Support Suppliers from China
Executive Summary
As global demand for ergonomic and orthopedic footwear solutions grows, sourcing high-quality arch support components from reliable Chinese manufacturers has become both a priority and a challenge. Market fragmentation, inconsistent quality standards, and due diligence overheads often delay time-to-market and inflate procurement costs.
SourcifyChina’s Verified Pro List for Arch Support Suppliers eliminates these barriers by providing procurement teams with pre-vetted, audit-ready suppliers who meet international compliance, quality control, and scalability benchmarks.
Why SourcifyChina’s Verified Pro List Saves Time & Reduces Risk
| Benefit | Impact on Procurement Efficiency |
|---|---|
| Pre-Vetted Suppliers | Eliminates 40–60 hours of initial supplier screening per project |
| Onsite Factory Audits | Verified production capacity, quality control systems, and export experience |
| Compliance Documentation | Suppliers provide ISO, BSCI, or equivalent certifications on request |
| Reference Clients & Case Studies | Real-world performance data to support supplier selection |
| Dedicated Sourcing Consultant | Single point of contact for RFQs, negotiations, and sample coordination |
Average Time Saved: Procurement cycles reduced by 30–50% compared to traditional sourcing methods.
Call to Action: Accelerate Your Sourcing in 2026
In a competitive landscape where speed and reliability define supply chain success, relying on unverified suppliers is no longer viable. SourcifyChina delivers precision, transparency, and speed in one streamlined solution.
By leveraging our Verified Pro List for Arch Support Suppliers, your team gains immediate access to trusted manufacturers—reducing onboarding time, minimizing quality risks, and ensuring faster time-to-market.
Take the Next Step Today
Contact our Sourcing Support Team to receive your customized shortlist of verified arch support suppliers—complete with audit summaries, MOQs, lead times, and pricing benchmarks.
📧 Email: [email protected]
📱 WhatsApp: +86 159 5127 6160
Responds within 2 business hours. All inquiries confidential.
SourcifyChina – Your Verified Gateway to China Manufacturing Excellence
Trusted by 320+ global brands in footwear, orthotics, and wellness sectors.
🧮 Landed Cost Calculator
Estimate your total import cost from China.


