The global sphygmomanometer market is experiencing steady growth, driven by rising hypertension prevalence, increasing demand for home healthcare devices, and expanding telemedicine services. According to Grand View Research, the global sphygmomanometer market size was valued at USD 689.2 million in 2022 and is expected to expand at a compound annual growth rate (CAGR) of 7.1% from 2023 to 2030. Aneroid manometers, valued for their portability, durability, and lack of reliance on electricity, continue to play a critical role in clinical and ambulatory settings, particularly in regions with limited access to advanced medical infrastructure. As healthcare providers prioritize accuracy and ease of use, leading manufacturers are investing in ergonomic designs, calibration precision, and mercury-free alternatives to meet regulatory and environmental standards. Against this backdrop, the following six companies have emerged as top aneroid manometer manufacturers, demonstrating strong market presence, innovation, and global distribution networks.
Top 6 Aneroid Manometer Manufacturers 2026
(Ranked by Factory Capability & Trust Score)
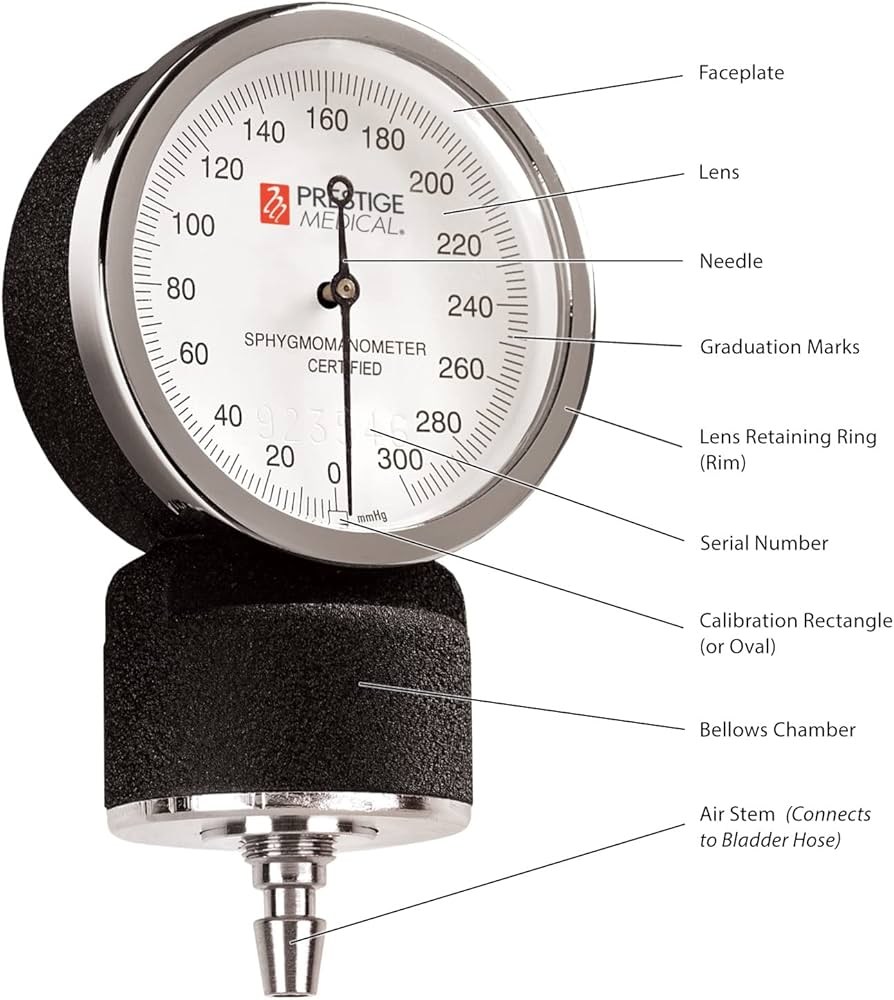
#1 Aneroid sphygmomanometer
Domain Est. 1997
Website: medicalexpo.com
Key Highlights: A compact aneroid sphygmomanometer designed for emergency purposes which provided in five sizes of cuff (Infant, Child, Adult, Large Adult and Thigh)….
#2 Palm Aneroid Sphygmomanometers
Domain Est. 1998
Website: adctoday.com
Key Highlights: American Diagnostic Corporation’s (ADC) manometers are engineered by Nissei, one of the oldest and most respected manufactures of aneroid gauges….
#3 Aneroid Sphygmomanometers
Domain Est. 1999
Website: kermamedical.com
Key Highlights: Premium Aneroid Sphygmomanometer · Black enamel 300mmHg no-pin stop manometer · Navy blue nylon cuff with range markings, artery label and gauge holder · Dip ……
#4 Calibra® Pro Sphygmomanometer
Domain Est. 2003
#5 High quality manual aneroid sphygmomanometer blood pressure …
Domain Est. 2018
Website: ticarehealth.com
Key Highlights: Our manual aneroid sphygmomanometers are designed to provide accurate and consistent readings, even in noisy or busy environments. They are lightweight, ……
#6 Riester Aneroid Sphygmomanometers
Website: riester.de
Key Highlights: A state of the art, aneroid, palm style sphygmomanometer available as 1 or 2 tube versions with disinfectable, latex-free, one-piece cuffs….
Expert Sourcing Insights for Aneroid Manometer

H2: 2026 Market Trends for Aneroid Sphygmomanometers
The global market for aneroid sphygmomanometers—mechanical blood pressure measurement devices widely used in clinical and home healthcare settings—is expected to undergo notable shifts by 2026, driven by technological advancements, evolving healthcare needs, and regulatory changes. Despite increasing adoption of digital and smart blood pressure monitors, aneroid manometers continue to hold significant value, particularly in resource-limited and primary care environments.
1. Sustained Demand in Low- and Middle-Income Countries (LMICs)
A key trend shaping the 2026 market is the continued reliance on aneroid devices in developing regions. Due to their affordability, durability, and lack of dependence on electricity or batteries, aneroid sphygmomanometers remain a preferred choice in rural clinics and mobile health units across Africa, South Asia, and Latin America. As governments and NGOs expand primary healthcare infrastructure, procurement of cost-effective diagnostic tools like aneroid manometers is projected to remain steady.
2. Regulatory and Accuracy Standards Driving Innovation
Growing emphasis on measurement accuracy and device calibration is prompting manufacturers to enhance the reliability of aneroid models. By 2026, compliance with updated standards such as the AAMI/ESH/ISO 81060-2 is expected to become a market differentiator. Leading companies are investing in precision engineering, anti-shock valves, and tamper-proof designs to reduce measurement errors and prolong device lifespan.
3. Integration with Training and Education Initiatives
Medical training programs continue to use aneroid sphygmomanometers to teach auscultatory blood pressure techniques, reinforcing their role in clinical education. This educational stickiness supports sustained institutional demand. In 2026, partnerships between device manufacturers and medical schools or certification bodies are likely to grow, including bundled training kits and certification programs for proper usage.
4. Competitive Pressure from Digital Alternatives
The aneroid segment faces increasing competition from oscillometric digital monitors, especially in home healthcare and telemedicine applications. Smart devices offering Bluetooth connectivity, mobile app integration, and AI-based hypertension tracking are gaining market share. However, concerns about the accuracy of some automated devices in specific patient populations (e.g., arrhythmias) preserve a niche for skilled manual measurement using aneroid units.
5. Market Consolidation and Regional Manufacturing Shifts
By 2026, the aneroid manometer market is expected to see consolidation among mid-tier manufacturers, with larger medical device companies acquiring brands to maintain a presence in the manual diagnostics segment. Additionally, regional manufacturing in Asia (particularly China and India) will dominate supply chains, driven by cost efficiency and proximity to high-growth markets.
6. Sustainability and Reusability as a Market Advantage
With rising awareness of medical waste, the reusable nature of aneroid devices—versus disposable or short-life digital units—may position them as environmentally sustainable options. This could appeal to healthcare systems aiming to meet green procurement goals, providing a subtle but growing competitive edge.
In conclusion, while the aneroid sphygmomanometer market is mature and not expected to see explosive growth by 2026, it will maintain relevance through strategic positioning in cost-sensitive, educational, and accuracy-critical applications. Innovation in design, adherence to standards, and alignment with global health equity goals will be crucial for sustained market presence.

H2: Common Pitfalls When Sourcing Aneroid Manometers (Quality and Intellectual Property)
Sourcing aneroid manometers, especially for medical or precision applications, involves several risks related to product quality and intellectual property (IP). Being aware of these pitfalls helps ensure reliable performance, regulatory compliance, and legal safety. Below are key challenges to consider:
1. Poor Manufacturing Quality
Many low-cost suppliers, particularly from unverified manufacturers, produce aneroid manometers with inconsistent calibration, substandard materials, or poor mechanical durability. This leads to inaccurate blood pressure readings, posing serious risks in clinical settings. Look for ISO 13485 certification and adherence to ANSI/AAMI/ISO 81060 standards to ensure quality.
2. Inadequate Calibration and Traceability
Some suppliers offer manometers without proper calibration documentation or traceable standards (e.g., NIST). Without this, devices cannot be trusted for diagnostic use. Always request calibration certificates and verify pre-shipment testing protocols.
3. Counterfeit or Clone Devices
Unscrupulous suppliers may copy branded designs (e.g., Welch Allyn) without authorization, infringing on intellectual property rights. These counterfeit products often mimic appearance but lack performance reliability and legal compliance. Conduct due diligence on OEMs and request proof of IP ownership or licensing.
4. Lack of Regulatory Compliance
Aneroid manometers are Class II medical devices in many regions (e.g., FDA in the U.S., CE in Europe). Sourcing from manufacturers without proper regulatory approvals can result in shipment seizures, legal penalties, or liability in case of device failure. Ensure the supplier has valid 510(k) clearances or CE Marking with Technical File support.
5. Hidden IP Infringement Risks
Even if a supplier appears legitimate, the design or internal mechanism may violate patents or trade secrets. This exposes the buyer to legal action, especially when importing or reselling. Conduct IP clearance searches and include IP indemnity clauses in procurement contracts.
6. Inconsistent After-Sales Support and Repairs
Low-quality suppliers often lack service networks for recalibration or part replacement. This impacts long-term usability and increases total cost of ownership. Prioritize vendors offering warranties, service centers, and spare parts availability.
7. Misleading Product Specifications
Some suppliers exaggerate accuracy, durability, or compliance claims. Independent verification through third-party testing or audits is essential before large-scale procurement.
To mitigate these risks, work with reputable, audited suppliers, request samples for validation, and involve legal and regulatory experts during the sourcing process.

Logistics & Compliance Guide for Aneroid Manometer
Product Classification and Regulatory Overview
Aneroid manometers are medical devices used to measure blood pressure manually. They are classified under medical device regulations in most jurisdictions. In the United States, they are regulated by the FDA as Class I or Class II devices depending on design and intended use, typically falling under 21 CFR 870.1100 (Blood Pressure Measuring Device). In the European Union, they must comply with the Medical Device Regulation (MDR) (EU) 2017/745 and carry the CE mark. Classification may vary by region, so local regulatory requirements must be verified prior to distribution.
Packaging and Labeling Requirements
Aneroid manometers must be packaged to prevent physical damage during transit, particularly to the gauge and inflation bulb. Packaging should be durable and include protective cushioning. Labels must include: device name, model number, manufacturer name and address, CE mark (if applicable), FDA registration number (for U.S. market), expiration date (if components degrade), and symbols per ISO 15223-1. Multilingual labeling may be required for international markets. All labeling must conform to local language and regulatory requirements.
Transportation and Storage Conditions
Shipments should avoid extreme temperatures, humidity, and physical shock. Store and transport in a dry, climate-controlled environment (typically 10°C to 40°C). Avoid prolonged exposure to direct sunlight or moisture, which may damage the mechanical components or cause rust. Stack packages according to weight limits to prevent crushing. Use carriers experienced in medical device logistics to ensure proper handling.
Import and Export Compliance
Exporting aneroid manometers requires compliance with international trade regulations. Obtain necessary export licenses if required by the country of origin. Ensure compliance with destination country regulations—such as Health Canada for Canada, TGA for Australia, or NMPA for China. Include accurate Harmonized System (HS) codes (e.g., 9018.12.00 for blood pressure monitors) on commercial invoices. Provide Certificates of Conformity, Free Sale Certificates, or other documentation as required by customs authorities.
Quality and Calibration Documentation
Each aneroid manometer should be accompanied by a calibration certificate indicating traceability to national standards (e.g., NIST in the U.S.). Maintain quality management system (QMS) compliance per ISO 13485. Records of manufacturing, testing, and calibration must be retained for regulatory audits. Distributors and importers should verify that devices are supplied with up-to-date documentation and that recalibration schedules are communicated to end users.
Post-Market Surveillance and Recalls
Establish a system for monitoring device performance in the field. Report adverse events or malfunctions to relevant regulatory bodies (e.g., FDA MedWatch, EU vigilance system). Maintain recall procedures in accordance with local regulations. Ensure traceability through batch/lot numbers and serial tracking. Communicate promptly with distributors and healthcare providers in the event of a field safety notice or recall.
Environmental and Disposal Considerations
Aneroid manometers are generally non-powered and do not contain hazardous materials like mercury (unlike older sphygmomanometers). However, they may contain metal and plastic components. Follow local regulations for disposal of medical devices. Encourage recycling where possible and provide guidance on environmentally responsible end-of-life handling. Comply with WEEE or similar directives if applicable.
Conclusion:
Sourcing an aneroid sphygmomanometer requires careful consideration of accuracy, durability, calibration standards, and user requirements. While aneroid manometers are portable, cost-effective, and do not rely on mercury—making them environmentally and safety-friendly—they must be regularly calibrated to ensure reliable blood pressure readings. When sourcing, it is essential to select devices from reputable manufacturers that comply with international standards such as ANSI, BHS, or the AAMI. Additionally, factors like ease of use, readability of the gauge, availability of replacement parts, and after-sales support should influence procurement decisions. For clinical settings, proper training on use and maintenance is crucial to avoid measurement errors. In conclusion, while aneroid manometers remain a practical choice for many healthcare environments, diligent sourcing and ongoing maintenance are key to ensuring consistent accuracy and patient safety.