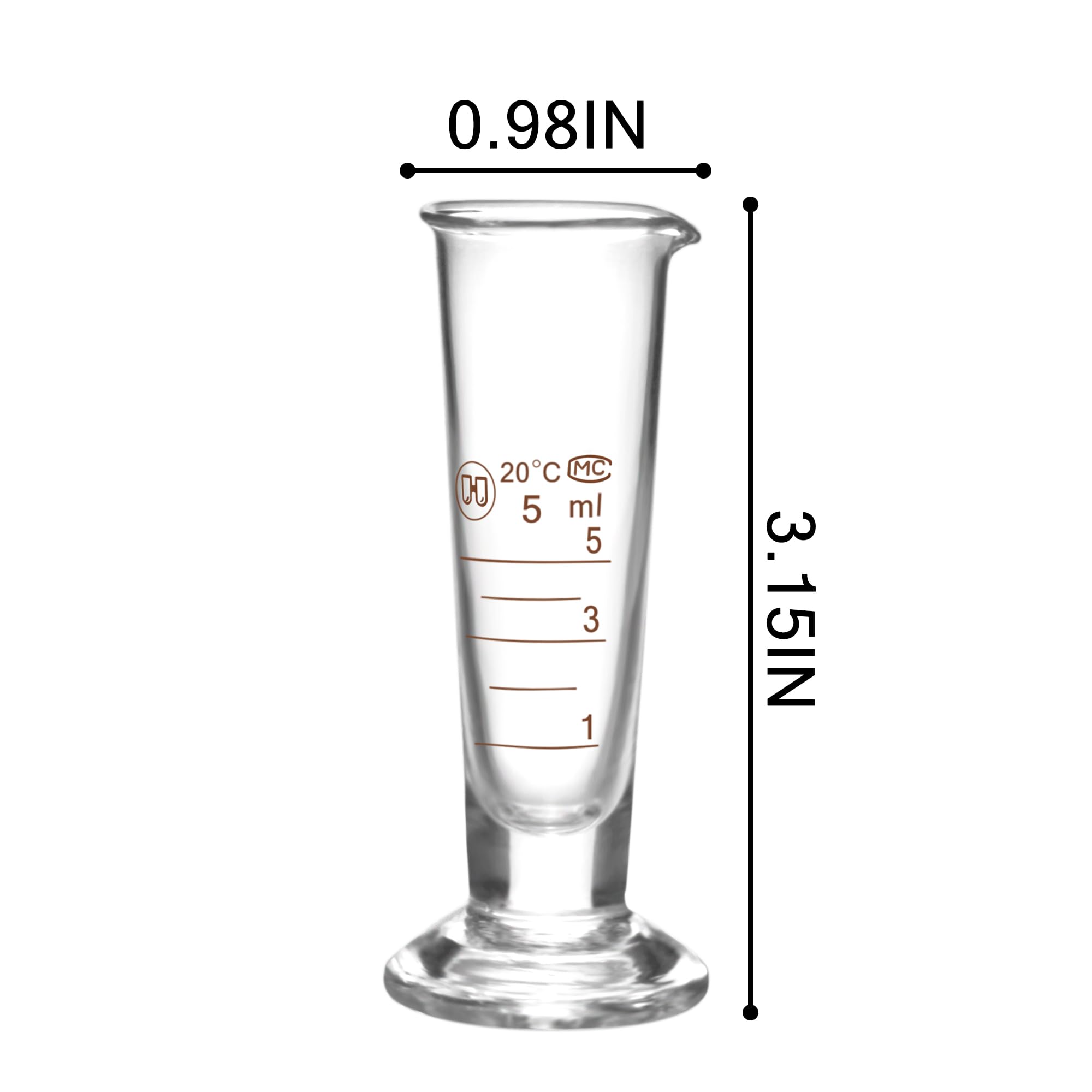
The global demand for small-volume packaging solutions, including 5ml cups, has seen steady growth driven by expanding applications in pharmaceuticals, diagnostics, food & beverage sampling, and laboratory testing. According to Mordor Intelligence, the global small-volume packaging market is projected to grow at a CAGR of over 5.8% from 2023 to 2028, fueled by rising emphasis on precision dosing, product sampling, and single-use convenience. Additionally, increased R&D spending in biotech and pharmaceutical sectors has amplified the need for reliable, sterile, and scalable container solutions like 5ml cups. As industries prioritize accuracy, contamination control, and sustainable materials, manufacturers with advanced production capabilities, strict compliance standards (e.g., ISO, FDA), and scalable output are gaining competitive advantage. Based on production volume, certifications, innovation in materials (such as PCR and bioplastics), and global distribution reach, we’ve identified the top nine 5ml cup manufacturers shaping this growing niche.
Top 9 5Ml Cup Manufacturers 2026
(Ranked by Factory Capability & Trust Score)
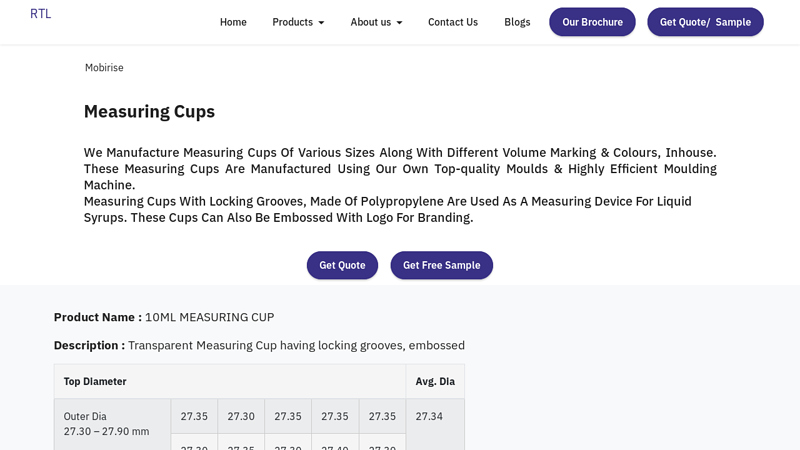
#1 Pharmaceutical Plastic Measuring Cups Manufacturers …
Domain Est. 2008
Website: rajatl.com
Key Highlights: Raja TL has emerged as the leading pharmaceutical measuring cup supplier over the years. We have been delivering Transparent measuring cups to measure liquid ……
#2 AP05 Measuring Cup Manufacturer from Nashik
Domain Est. 2011
Website: plasticbottlesmanufacturer.com
Key Highlights: Capacity: 5 ml · Material: Plastic · Application: Measure · Usage/Application: Chemical Laboratory · Brand: Akhil plast · Country of Origin: Made in India….
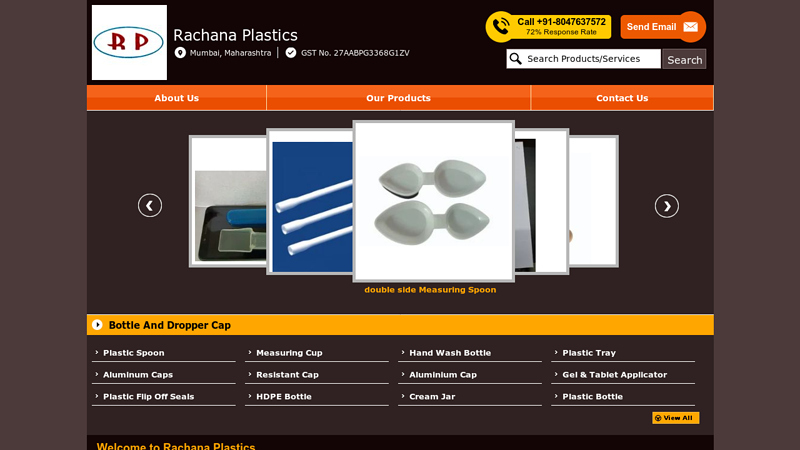
#3 Plastic Spoon and Measuring Cup Manufacturer
Domain Est. 2018
Website: rachanaplastics.com
Key Highlights: Rachana Plastics – Plastic Spoon, Measuring Cup & Hand Wash Bottle Manufacturer from Mumbai, Maharashtra, India. … 5ml Measuring Spoon · Rs 2 / Piece. Material: ……
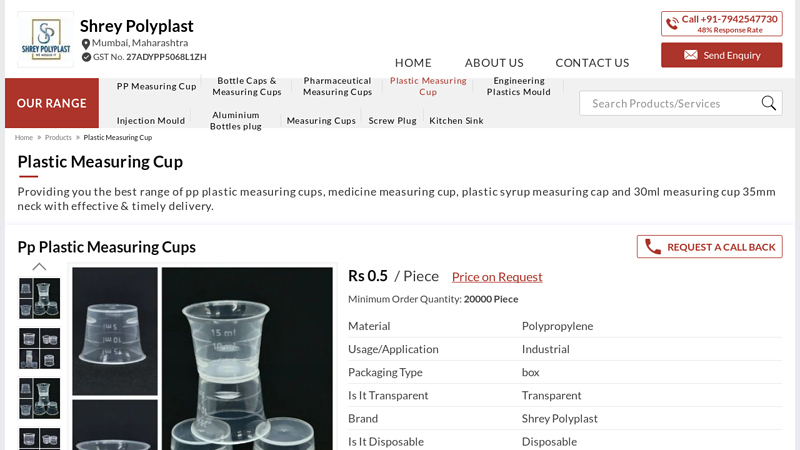
#4 Pp Plastic Measuring Cups Manufacturer from Mumbai
Domain Est. 2022
Website: shreypolyplast.com
Key Highlights: Plastic Syrup Measuring Cap · Capacity: 5 ml · Material: PP · Shape: Circular · Usage/Application: Syrups Medicine · Country of Origin: Made in India….
#5 Dosage Cups
Domain Est. 1999
Website: comar.com
Key Highlights: Catalog; Dosage Cups. Dosage Cups. PRODUCTS. Canisters. Closures. Dosage Cups. Dropper Assemblies. Dropper Squeeze Bottles. Fitments & Adapters. Jars….
#6 Resimix Mixing Cups
Domain Est. 2000
Website: osecompany.com
Key Highlights: 180-day returnsResimix Mixing Cups ; 7243-000. Desc: 5ml – Small, $9.95. Resimix Mixing Cups quantity. Add to Cart….
#7 5ml 22mm Measuring Cup High
Domain Est. 2011
Website: shakoplastick.com
Key Highlights: Discover the 5ml 22mm Measuring Cup, made from food-grade polypropylene with clear graduation markings. Perfect for pharmaceutical, chemical, agro, ……
#8 Measuring Cup and Spoon
Domain Est. 2015
Website: eastpharmatechnologies.com
Key Highlights: Measuring Cup and Spoon manufactured in East Pharma Technologies in clean room for Oral Dispensing of Medicine….
#9 Measuring Caps and Cups
Domain Est. 2018
Website: neelkanthpolymer.net
Key Highlights: We are offering here the Measuring Caps and Cups used primarily to measure the volume of liquid or bulk solid medicines especially for volumes from about 50 ml….
Expert Sourcing Insights for 5Ml Cup

H2: Analysis of 2026 Market Trends for 5ml Cups
The global market for 5ml cups—commonly used in pharmaceutical, cosmetic, laboratory, and food sampling applications—is projected to experience notable shifts by 2026, driven by evolving consumer demands, regulatory changes, and advancements in sustainable packaging. Below is a comprehensive analysis of key market trends expected to shape the 5ml cup industry in 2026.
-
Increased Demand in Healthcare and Diagnostics
The healthcare sector is a primary driver for 5ml cup usage, particularly in diagnostic testing, specimen collection, and single-dose medication delivery. By 2026, the expansion of personalized medicine, at-home testing kits, and point-of-care diagnostics will significantly boost demand. The rise in telehealth and decentralized testing models is expected to increase the need for small, standardized, and sterile containers like 5ml cups, especially for saliva, urine, and blood sample collection. -
Growth in Cosmetic and Skincare Sampling
The beauty and personal care industry continues to adopt 5ml cups for product sampling, travel kits, and clinical trials. With consumers increasingly demanding trial-sized products to test efficacy before full-size purchases, brands are investing in eco-friendly 5ml sample packaging. In 2026, expect innovation in tamper-evident, airless, and recyclable 5ml cups tailored for premium skincare formulations. -
Sustainability and Regulatory Pressure
Environmental regulations targeting single-use plastics are accelerating the shift toward biodegradable and compostable 5ml cups. By 2026, many regions—including the EU, Canada, and parts of the U.S.—will enforce stricter packaging mandates, pushing manufacturers to adopt plant-based PLA (polylactic acid), paper-based, or reusable alternatives. Brands emphasizing ESG (Environmental, Social, and Governance) goals will prioritize sustainable 5ml cup solutions to maintain consumer trust and regulatory compliance. -
Advancements in Material and Design Innovation
Material science developments are leading to enhanced barrier properties, improved clarity, and better chemical resistance in 5ml cups—critical for pharmaceutical and lab applications. Innovations such as antimicrobial coatings, QR code integration for traceability, and smart labeling (e.g., time-temperature indicators) are expected to become more prevalent by 2026, especially in cold chain and clinical trial logistics. -
E-Commerce and Direct-to-Consumer (DTC) Models
The rise of e-commerce platforms and DTC health and beauty brands is reshaping packaging needs. Compact, stackable, and lightweight 5ml cups are ideal for shipping efficiency and cost reduction. By 2026, expect customized branding, minimalistic design, and integration with automated filling and sealing systems to support scalable online distribution models. -
Regional Market Expansion
Asia-Pacific, particularly India and Southeast Asia, is projected to be the fastest-growing market for 5ml cups due to rising healthcare access, cosmetic industry growth, and expanding laboratory infrastructure. Meanwhile, North America and Europe will maintain strong market shares, driven by innovation and regulatory leadership in sustainable packaging. -
Consolidation and Strategic Partnerships
The competitive landscape will likely see increased consolidation among packaging manufacturers and partnerships between cup producers and biotech or cosmetic firms. These collaborations will focus on co-developing application-specific 5ml cups with enhanced functionality, sterility, and branding potential.
Conclusion
By 2026, the 5ml cup market will be characterized by a convergence of sustainability, technological innovation, and sector-specific customization. Growth will be fueled by healthcare diagnostics, beauty sampling, and environmental regulations, with a strong emphasis on eco-design and smart packaging. Companies that proactively adapt to these trends—through material innovation, regulatory compliance, and customer-centric design—will be best positioned to capture market share in this niche but rapidly evolving segment.
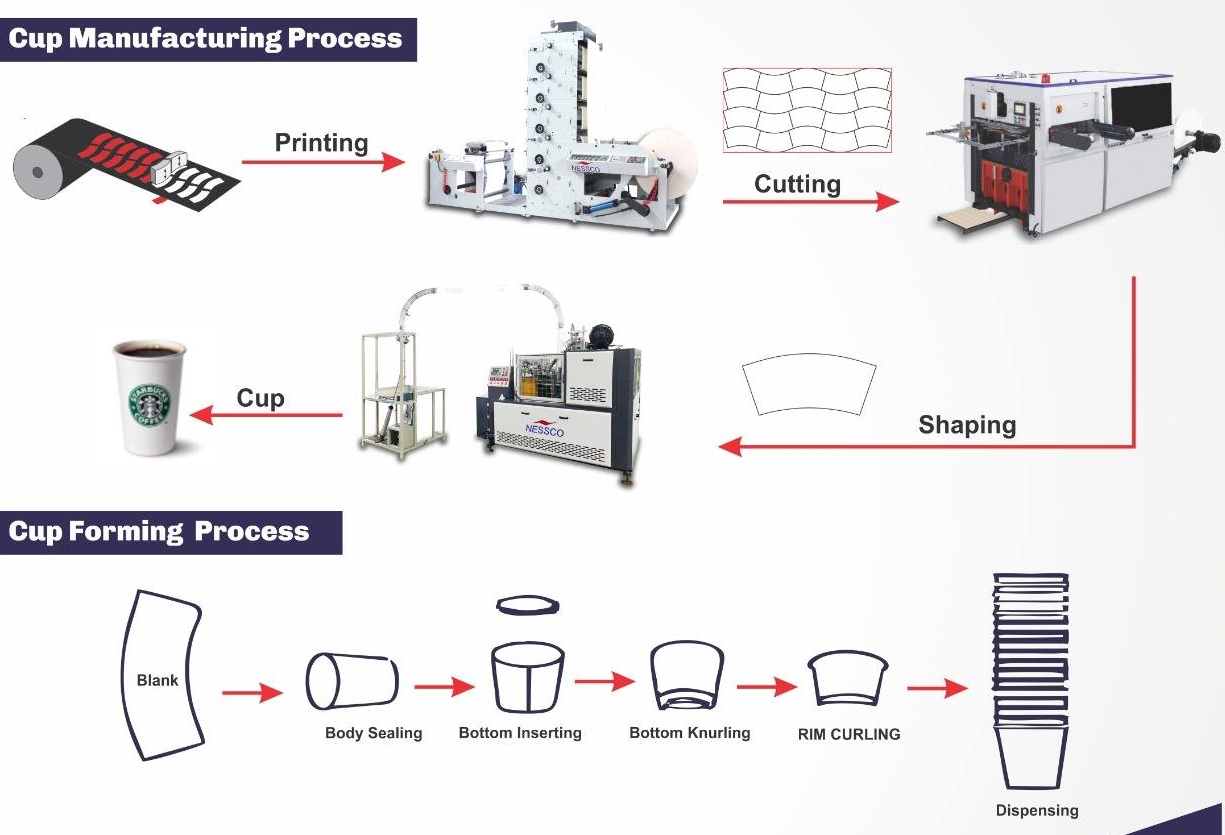
Common Pitfalls When Sourcing 5ml Cups: Quality and Intellectual Property Concerns
Sourcing 5ml cups—commonly used in cosmetics, pharmaceuticals, food sampling, and lab settings—can present significant challenges if not managed carefully. Two critical areas where buyers often encounter issues are product quality and intellectual property (IP) risks. Overlooking these can lead to supply chain disruptions, customer dissatisfaction, legal disputes, and reputational damage.
Quality-Related Pitfalls
-
Inconsistent Material Quality
Suppliers may use substandard or recycled plastics that compromise the cup’s clarity, strength, or chemical resistance. This can lead to leaks, breakage, or contamination, especially when holding oils, solvents, or reactive substances. -
Dimensional Inaccuracy
Poor mold precision or manufacturing control can result in cups that are slightly under or over 5ml in volume. This affects dosing accuracy, particularly critical in pharmaceutical or diagnostic applications. -
Lack of Regulatory Compliance
Many 5ml cups are used for food or medical applications, requiring compliance with standards like FDA, EU 10/2011, or USP Class VI. Sourcing from suppliers without proper certifications risks non-compliance and product recalls. -
Poor Seal or Lid Compatibility
Cups may not properly fit standard lids or sealing films, leading to spills or compromised sterility. This is especially problematic in automated filling lines where tight tolerances are required. -
Inadequate Packaging and Handling
Bulk-packed cups may arrive scratched, nested improperly, or contaminated due to poor handling during transit or storage, affecting usability and perceived quality.
Intellectual Property (IP) Pitfalls
-
Copying Branded or Patented Designs
Some 5ml cup designs (e.g., specific shapes, stackable features, or dispensing mechanisms) are protected by patents or design rights. Sourcing look-alike products from third parties may constitute IP infringement, exposing the buyer to legal action. -
Unauthorized Use of Trademarks or Logos
Suppliers may offer to print branded logos or use trademarked design elements without permission. Even if the buyer requests this, they can still be held liable for trademark infringement. -
Design Theft and Reverse Engineering
When working with OEM suppliers to create custom 5ml cups, there is a risk that the supplier may replicate and sell the design to competitors—especially in regions with weak IP enforcement. -
Lack of IP Clauses in Contracts
Failure to include clear IP ownership terms in sourcing agreements can result in disputes over who owns the design, molds, or tooling—potentially limiting exclusivity or future production options. -
Grey Market or Counterfeit Goods
Some suppliers may offer seemingly legitimate branded cups at unusually low prices, which may be counterfeit or diverted from authorized distribution channels. Purchasing such goods may expose the buyer to IP liability and quality risks.
Mitigation Strategies
- Conduct thorough supplier audits, including factory inspections and material certifications.
- Require compliance documentation (e.g., FDA letters, test reports).
- Use NDAs and clear contracts that assign IP rights and prohibit unauthorized replication.
- Work with legal counsel to verify that custom or branded designs do not infringe existing IP.
- Source from reputable manufacturers with proven track records in regulated industries.
Avoiding these pitfalls ensures reliable supply, protects brand integrity, and reduces legal and operational risks in the sourcing process.
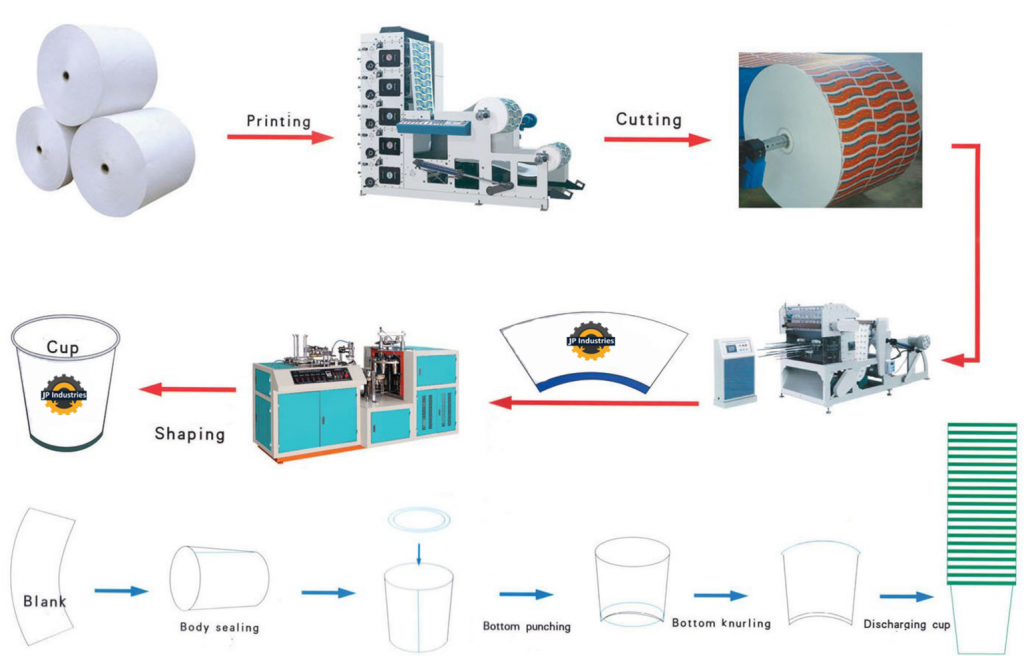
Logistics & Compliance Guide for 5ml Cup
This guide outlines the key logistics considerations and compliance requirements for handling, storing, transporting, and disposing of 5ml cups, which are commonly used in laboratories, medical facilities, food sampling, and cosmetic testing. Adhering to these guidelines ensures safety, regulatory compliance, and efficient operations.
Product Specifications and Labeling
Ensure all 5ml cups meet defined physical and material standards. Cups should be clearly labeled with:
– Manufacturer name and lot number
– Material type (e.g., polypropylene, PET)
– Volume marking (5ml) clearly visible
– Sterility status (if applicable)
– Single-use indication, if relevant
– Any hazard symbols or handling instructions (e.g., “Do not microwave”)
Labels must be durable and resistant to common lab solvents, moisture, and temperature changes.
Storage Conditions
Store 5ml cups in a clean, dry, and temperature-controlled environment:
– Avoid direct sunlight and UV exposure
– Maintain ambient temperatures between 15°C and 25°C
– Keep away from strong chemicals, fumes, or sources of contamination
– Stack neatly to prevent deformation or damage
– Protect from dust and pests using sealed packaging or cabinets
For sterile cups, maintain original sealed packaging until point of use.
Transportation Requirements
During shipping and handling:
– Use sturdy, contaminant-free packaging to prevent crushing or puncturing
– Secure loads to prevent shifting during transit
– Follow regulations for transporting laboratory or medical supplies if applicable (e.g., IATA for air freight)
– If transporting biohazard-contaminated cups, use UN-certified secondary containment and comply with DOT/ADR/IATA dangerous goods regulations
– Maintain cold chain if cups contain temperature-sensitive samples
Document chain of custody when required (e.g., forensic or clinical samples).
Regulatory Compliance
Adhere to relevant local, national, and international standards:
– FDA 21 CFR: For cups used in food, drug, or medical device applications
– EU REACH and RoHS: Ensure materials are free from restricted substances
– ISO 10993: If cups contact biological tissues (biocompatibility)
– CLP Regulation (EU): For proper classification and labeling of hazardous contents
– OSHA Hazard Communication Standard (HCS): When handling hazardous materials in cups
Verify that suppliers provide Certificates of Conformance (CoC) or Compliance (CoA) as needed.
Waste Disposal and Environmental Responsibility
Dispose of used 5ml cups according to their contamination level:
– Non-hazardous waste: Recycle if material is recyclable (e.g., PP, PET) and uncontaminated
– Biohazardous waste: Autoclave or incinerate following local biosafety protocols
– Chemical-contaminated waste: Dispose as hazardous waste per EPA or equivalent regulations
– Sharps or puncture risks: Use puncture-resistant containers if applicable
Follow local environmental regulations and prioritize reusable or biodegradable alternatives where feasible.
Training and Documentation
Ensure all personnel are trained on:
– Proper handling and storage procedures
– Spill response and personal protective equipment (PPE) requirements
– Waste segregation and disposal protocols
– Regulatory compliance relevant to their role
Maintain records of training, inventory logs, safety data sheets (SDS), and disposal manifests for audit readiness.
Quality Assurance and Audits
Conduct regular audits to verify compliance with internal SOPs and external regulations. Inspect:
– Cup integrity upon receipt
– Storage conditions and labeling accuracy
– Waste disposal practices
– Documentation completeness
Address non-conformances promptly and update procedures as needed.
By following this guide, organizations can ensure the safe, compliant, and efficient use of 5ml cups across logistics and operational workflows.
Conclusion for Sourcing 5ml Cups
After a thorough evaluation of various suppliers, materials, cost structures, quality standards, and lead times, sourcing 5ml cups can be effectively achieved by partnering with a reliable, certified manufacturer that meets both regulatory and operational requirements. Key considerations such as material safety (e.g., food-grade or lab-grade plastics), product consistency, minimum order quantities, and sustainable practices have been assessed to ensure optimal value and performance.
In conclusion, the recommended sourcing strategy involves selecting a supplier that offers high-quality 5ml cups—preferably made from PET, PS, or biodegradable materials—supported by competitive pricing, scalable production capacity, and timely delivery. Establishing a long-term partnership with such a supplier will ensure consistency in supply, compliance with industry standards, and cost-efficiency, ultimately supporting our operational goals in applications such as sampling, diagnostics, food service, or laboratory use. Ongoing supplier performance monitoring and regular market assessments will further enhance sourcing effectiveness and risk mitigation.