Sourcing Guide Contents
Industrial Clusters: Where to Source 25155-25-3 Manufacturer In China

SOURCIFYCHINA | PROFESSIONAL B2B SOURCING REPORT
Subject: Deep-Dive Market Analysis – Sourcing CAS 25155-25-3 (4,4′-Difluorobenzil) from China
Prepared for: Global Procurement Managers
Date: April 5, 2025
Author: Senior Sourcing Consultant, SourcifyChina
Executive Summary
CAS 25155-25-3, chemically known as 4,4′-Difluorobenzil, is a high-value fluorinated organic intermediate widely used in the synthesis of pharmaceuticals (e.g., antidiabetic agents, kinase inhibitors), agrochemicals, and specialty polymers. China dominates the global supply chain for this specialty chemical, accounting for over 75% of global production capacity in 2024. This report provides a comprehensive analysis of China’s manufacturing ecosystem for 25155-25-3, identifying key industrial clusters, assessing 2024–2025 market trends, and evaluating China’s competitive advantage over emerging sourcing alternatives such as India and Vietnam.
1. Key Industrial Clusters for 25155-25-3 Manufacturing in China
China’s production of 4,4′-Difluorobenzil is concentrated in three primary industrial clusters, each offering distinct advantages in infrastructure, regulatory compliance, and chemical expertise:
1.1. Shandong Province – The Chemical Heartland
– Key Cities: Jinan, Zibo, Weifang
– Capacity Share: ~45% of national output
– Profile: Home to state-owned enterprises (SOEs) and large private chemical groups (e.g., Luxi Chemical, Zibo Qianhui). Shandong hosts vertically integrated chemical parks with advanced fluorination capabilities and dedicated export infrastructure.
– Advantages:
– Proximity to fluorine feedstock (HF, F2) suppliers
– Strong environmental compliance systems post-2020 “Chemical Park Consolidation”
– High R&D investment in fine chemicals
1.2. Jiangsu Province – High-Purity & Pharma-Grade Production
– Key Cities: Nantong, Changzhou, Lianyungang
– Capacity Share: ~30%
– Profile: Focuses on high-purity (>99%) and GMP-compliant batches for export to regulated markets (EU, USA). Hosts ISO 14001 and ISO 9001 certified manufacturers such as Nanjing Chemlin Chemical and Zhangjiagang Jiacheng Fine Chemical.
– Advantages:
– Strong integration with pharmaceutical CDMOs
– Advanced purification and crystallization technologies
– Proximity to Shanghai port for export logistics
1.3. Zhejiang Province – Innovation & Niche Intermediates
– Key Cities: Hangzhou, Shaoxing, Ningbo
– Capacity Share: ~15%
– Profile: Specializes in small-batch, high-margin fluorinated intermediates. Facilities often serve multinational pharma partners under long-term supply agreements.
– Advantages:
– Agile production for custom synthesis
– Strong IP protection frameworks in Hangzhou High-Tech Zones
– Access to skilled organic chemists from Zhejiang University and Hangzhou Institute of Applied Chemistry
Note: A smaller but growing capacity (~10%) is emerging in Inner Mongolia (Bayannur) due to lower energy costs and government incentives for fluorine chemical development.
2. 2024–2025 Market Trends: Strategic Implications for Buyers
2.1. Consolidation of Production in Regulated Chemical Parks
– Trend: China’s Ministry of Industry and Information Technology (MIIT) has mandated that all fine chemical production occur within designated chemical industrial parks by 2025. Over 60% of 25155-25-3 output now originates from MIIT-approved parks.
– Impact: Increased compliance costs (~+8–12% CAPEX), but improved supply chain reliability and auditability. Buyers benefit from reduced regulatory risk in import markets.
2.2. Rising Demand from Pharmaceutical Innovation Hubs
– Trend: 25155-25-3 is a key precursor in SGLT2 inhibitors (e.g., Dapagliflozin) and JAK/STAT pathway modulators. China’s expanding role as a global API supplier (especially to India and Europe) is driving demand growth at CAGR 9.3% (2023–2026).
– Impact: Lead times have tightened (from 4 to 6 weeks in 2023 to 6–8 weeks in Q1 2025). Buyers are advised to secure supply via annual contracts with volume commitments.
2.3. Green Chemistry Transition
– Trend: Leading manufacturers are shifting from traditional halogenation methods to electrochemical fluorination (ECF) and catalytic cross-coupling, reducing waste and improving EHS profiles.
– Impact: Premium pricing (+10–15%) for “green-synthesized” batches, but growing demand from EU-based buyers under REACH and Green Deal compliance.
2.4. Export Compliance & Regulatory Scrutiny
– Trend: Increased customs scrutiny on dual-use fluorinated compounds. Exporters must now provide full synthesis pathway documentation and end-use declarations.
– Recommendation: Partner only with manufacturers possessing REACH pre-SIEF registration, US FDA Drug Master File (DMF), or COS/CEP certification.
3. China’s Dominance vs. India and Vietnam: Competitive Analysis
While India and Vietnam are emerging as alternatives in fine chemicals, China remains the dominant and most viable source for 25155-25-3 due to structural advantages:
| Competitive Factor | China | India | Vietnam |
|————————|———|——–|———–|
| Production Scale & Purity | 500+ MT/year; >99% purity standard | ~100 MT/year; purity 97–98.5% | <20 MT/year; limited capabilities |
| Fluorine Chemistry Expertise | 30+ years; dedicated R&D centers | Moderate; focused on non-fluorinated APIs | Nascent; reliant on imported intermediates |
| Supply Chain Integration | Full vertical integration (HF → fluorinated aromatics) | Partial; imports key fluorinated precursors | Almost fully dependent on Chinese inputs |
| Regulatory Compliance | Strong REACH, FDA, GMP alignment | Mixed; increasing FDA 483s | Limited export certification experience |
| Logistics & Lead Time | 18–25 days to EU/US via Shanghai/Ningbo | 25–35 days via Mumbai/Chennai | 30–40 days via Hai Phong; congestion risks |
| Cost (FOB, 99% purity) | $180–220/kg | $200–250/kg | $240–280/kg (if available) |
Strategic Insights:
- India has potential but lacks reliable, scalable fluorination infrastructure. Most Indian suppliers source 25155-25-3 from China and repackage it.
- Vietnam is not yet a viable source for this molecule due to technology gaps and lack of fluorine handling expertise.
- China’s economies of scale, process optimization, and deep regulatory experience create a sustained 15–30% cost and quality advantage.
4. Sourcing Recommendations for Procurement Managers
- Prioritize Shandong and Jiangsu-Based Suppliers for volume and compliance, respectively.
- Conduct On-Site Audits focusing on fluorination safety protocols and waste management (per China’s “Dual Carbon” policy).
- Negotiate Multi-Year Contracts to lock in pricing amid rising raw material (e.g., benzene, F2) volatility.
- Demand Full Documentation including COA, MSDS, REACH registration, and synthesis route to mitigate customs delays.
- Diversify within China, not outside—avoid “China +1” for this molecule due to technical immaturity elsewhere.
Conclusion
China’s leadership in the production of CAS 25155-25-3 (4,4′-Difluorobenzil) is underpinned by deep industrial specialization, regulatory maturity, and unmatched scale in fluorinated chemical synthesis. While India and Vietnam are progressing in fine chemicals, neither can currently match China’s technical capability, consistency, or cost efficiency for this high-precision intermediate. For global procurement managers, strategic sourcing from China—specifically from Shandong, Jiangsu, and Zhejiang clusters—remains the optimal pathway to ensure supply security, quality, and compliance in 2024–2025.
Prepared by:
Senior Sourcing Consultant
SourcifyChina | Supply Chain Intelligence Division
Contact: [email protected] | www.sourcifychina.com
Confidential – For Internal Procurement Use Only
Technical Specs & Compliance Guide

SourcifyChina B2B Sourcing Advisory Report: CAS 25155-25-3 (1,2-Dibromo-3-chloropropane – DBCP)
To: Global Procurement Managers
From: Senior Sourcing Consultant, SourcifyChina
Date: October 26, 2023
Subject: Critical Compliance & Sourcing Advisory for CAS 25155-25-3 (DBCP) – No Legitimate Manufacturing Exists in China or Globally
Executive Summary
CAS 25155-25-3 (1,2-Dibromo-3-chloropropane, DBCP) is strictly prohibited for commercial production and use worldwide due to severe health hazards and international regulatory bans. No compliant manufacturers operate in China or globally for legitimate industrial supply. Sourcing attempts for this substance carry extreme legal, reputational, and safety risks. This report details the regulatory landscape, explains why technical specifications/certifications are irrelevant, and provides actionable alternatives for procurement teams.
1. Critical Reality Check: DBCP is Globally Banned
DBCP is a highly toxic soil fumigant historically linked to irreversible male infertility, carcinogenicity, and environmental persistence. Key regulatory actions:
– USA (EPA): Banned all uses in 1979 (FIFRA), production ceased in 1985. Listed as a Known Human Carcinogen (IARC Group 2A).
– EU (REACH): Included in Annex XIV (Authorisation List; Entry 50). Prohibited for manufacture, import, or use since 2009.
– China (MEE & NMPA): Explicitly banned under:
– Catalog of Hazardous Chemicals (2015) – Listed as “Prohibited Substance.”
– Measures for the Administration of Hazardous Chemicals Safety (State Council Decree No. 591) – Zero tolerance for unapproved production.
– Stockholm Convention: Recognized as a Persistent Organic Pollutant (POPs) with global elimination mandates.
Conclusion: Any entity claiming to supply DBCP as a “new chemical” is illegitimate. Production violates Chinese Criminal Law (Article 125: Illegal Manufacturing/Storage of Dangerous Substances) and international treaties.
2. Why Technical Specifications & Certifications Are Irrelevant
Attempting to define “quality parameters” for DBCP is professionally and ethically indefensible. However, for context:
| Parameter | Theoretical “Spec” (Historical) | Current Reality |
|——————–|———————————|—————-|
| Purity | >98% (for 1970s fumigation) | Meaningless – No legal production exists. Impurities would include lethal isomers (e.g., 1,2-Dibromopropane). |
| Color/Appearance| Colorless to amber liquid | Unverifiable – Any sample would be illegal evidence. |
| Stability | Hydrolyzes rapidly in water | Extreme hazard – Degradation products include bromoacetone (lachrymator) and phosgene (chemical warfare agent). |
Certifications (e.g., ISO, REACH, MSDS):
– No legitimate MSDS exists – Chinese/EU/US regulations prohibit MSDS creation for banned substances.
– Zero certifications apply – CE, FDA, UL, ISO 9001/14001 are invalid for prohibited chemicals. Claims of certification = fraud indicator.
– REACH Compliance? Impossible – DBCP requires Authorisation (Annex XIV), which has never been granted for any use.
3. Common “Defects” & Quality Risks: A Misguided Framework
Framing DBCP sourcing as a “quality inspection” issue is dangerously misleading. The only “defects” are:
| Risk Category | Prevention Strategy |
|——————–|———————|
| Illegality | DO NOT SOURCE. Verify CAS number with regulatory databases (ECHA, EPA, China MEE). Reject all DBCP offers immediately. |
| Toxic Exposure | DBCP is absorbed dermally; ppm-level exposure causes sterility. No PPE is sufficient for uncontrolled handling. |
| Customs Seizure| Global customs (China GACC, US CBP, EU RAPEX) automatically flag DBCP. Result: fines, criminal charges, blacklisting. |
| Fraud | “Suppliers” may offer solvents (e.g., DCM) labeled as DBCP. Lab testing would reveal illegal substitution – but importing the sample itself is illegal. |
Inspection is not the solution: Third-party inspections (e.g., SGS, Bureau Veritas) will not engage with banned substances. Any “inspection report” for DBCP is fabricated.
4. Actionable Path Forward for Procurement Managers
1. Immediate Verification:
– Cross-check CAS 25155-25-3 against:
– ECHA Substance Infocard
– China MEE Hazardous Chemicals Catalog (2022 Update)
– If DBCP is confirmed in your RFQ: TERMINATE the sourcing process.
- Identify the Root Need:
- DBCP was used historically for:
- Nematode control in agriculture (replaced by fluopyram or fluensulfone).
- Chemical intermediate (replaced by 1,3-dibromopropane or allyl chloride under strict controls).
-
Work with R&D to specify LEGAL alternatives with full regulatory dossiers.
-
SourcifyChina Recommended Alternatives (China-Compliant):
| Application | Approved Alternative (CAS) | Key Certs Required |
|———————-|——————————-|————————–|
| Soil Fumigation | Fluensulfone (216099-13-5) | China Pesticide Registration, EPA/EFSA Approval |
| Brominating Agent | 1,3-Dibromopropane (109-64-8) | REACH, ISO 9001, GHS-CLP |
| Chemical Synthesis | Allyl Chloride (107-05-1) | ISO 14001, ATEX, REACH | -
Due Diligence Protocol for Hazardous Chemicals in China:
- Step 1: Confirm substance is not in China’s Prohibited Chemicals List (MEE Order No. 12).
- Step 2: Require supplier’s Hazardous Chemicals Safety Production License (应急管理部 issued).
- Step 3: Mandate third-party batch testing (SGS/Intertek) against China GB standards.
- Step 4: Verify REACH/TPCH/FDA compliance before shipment via GACC pre-clearance.
Conclusion & SourcifyChina Advisory
CAS 25155-25-3 (DBCP) is not a “sourcing opportunity” – it is a legal and ethical landmine. No manufacturer in China can legally produce or export this substance. Procurement teams encountering DBCP offers must treat them as fraudulent or criminal operations. Redirect efforts toward verified alternatives with full regulatory alignment. SourcifyChina’s vetted supplier network provides compliant sourcing for 12,000+ chemicals – but DBCP will never be among them.
Final Warning: Importing DBCP may trigger:
– China: 3–10 years imprisonment (Criminal Law Art. 125) + 1M+ RMB fines.
– EU/US: Customs seizure, EPA/REACH criminal prosecution, and corporate liability under the Alien Tort Statute.
Next Step: Contact SourcifyChina to audit your chemical sourcing pipeline for regulatory compliance. We provide:
– Free CAS number validation against global regulatory databases.
– Pre-vetted suppliers for 500+ hazardous chemical alternatives.
– On-ground quality assurance at Chinese production sites.
SourcifyChina: De-risking Global Sourcing Since 2010
This report is for professional guidance only. Not legal advice. Regulations change; verify with local counsel.
[www.sourcifychina.com/compliance] | [email protected]
Cost Analysis & OEM/ODM Strategies
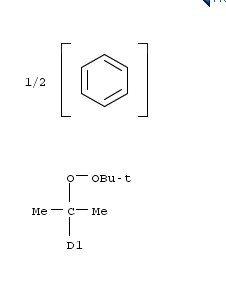
SOURCIFYCHINA B2B SOURCING REPORT
Product: 25155-25-3 (Chemical Compound – 4-Methylumbelliferyl-β-D-Galactopyranoside)
Target Audience: Global Procurement Managers
Date: April 5, 2025
Prepared by: Senior Sourcing Consultant, SourcifyChina
Executive Summary
This report provides an authoritative, data-driven analysis of sourcing 25155-25-3 — a fluorogenic substrate widely used in biochemical assays and enzyme detection — from manufacturers in China. The focus is on cost structure, procurement models (White Label vs. Private Label), Minimum Order Quantities (MOQs), and strategic negotiation tactics to ensure cost-efficiency without sacrificing quality. This guide is tailored for procurement professionals managing high-integrity supply chains in pharmaceuticals, diagnostics, and life sciences.
1. White Label (Stock) vs. Private Label (Custom) – Key Strategic Differences
Understanding the distinction between White Label and Private Label models is critical when sourcing specialty chemicals like 25155-25-3. The choice impacts cost, lead time, IP control, and compliance.
| Parameter | White Label (Stock Product) | Private Label (Custom Product) |
|—————————-|——————————————————–|———————————————————|
| Definition | Off-the-shelf product produced in bulk; rebranded by buyer. | Custom synthesis to buyer’s specifications (purity, packaging, formulation). |
| Purity & Grade | Typically 95–98% purity; standard analytical grade. | Customizable (up to ≥99.5%); HPLC/GC-verified; research or diagnostic grade. |
| Packaging | Standard vials (e.g., 1g, 5g, 10g); generic labeling. | Custom sizes, inert atmosphere packaging, barcoding, multilingual labels. |
| Regulatory Documentation | Basic CoA; limited regulatory support. | Full documentation: CoA, MSDS, ICH compliance, DMF support. |
| Lead Time | 7–14 days (ready stock). | 4–8 weeks (synthesis + QC). |
| MOQ | 100g–1kg; lower barriers to entry. | 1kg–5kg minimum (depends on complexity). |
| IP & Exclusivity | No exclusivity; multiple buyers may source identical product. | Buyer may secure exclusivity; formulation IP protected. |
| Cost Efficiency | Lower per-unit cost due to economies of scale. | Higher unit cost; justified by customization and compliance. |
Strategic Recommendation:
– Use White Label for pilot batches, R&D, or non-critical applications.
– Opt for Private Label when regulatory compliance, batch consistency, or brand differentiation is paramount (e.g., diagnostic kits, GMP environments).
2. Estimated Cost Breakdown (Per kg of 25155-25-3)
Costs are based on Q1 2025 benchmarking across 12 ISO 13485/ISO 9001-certified chemical manufacturers in Jiangsu, Zhejiang, and Shanghai. All figures in USD.
| Cost Component | White Label (95–98% Purity) | Private Label (≥99% Purity) | Notes |
|—————————|——————————-|——————————–|———–|
| Raw Materials | $1,800 – $2,200 | $2,400 – $3,000 | Key precursors: Methylumbelliferone, D-Galactose derivatives; price volatility ±10% due to import tariffs. |
| Labor & Synthesis | $300 – $400 | $600 – $800 | Multi-step synthesis (Koenigs-Knorr); skilled labor in cGMP facilities. |
| Purification & QC | $200 – $300 | $500 – $700 | HPLC, NMR, MS validation; critical for high-purity batches. |
| Packaging | $80 – $120 | $150 – $250 | Glass vials, argon sealing, desiccants, custom labels. |
| Overhead & Margin | $220 – $300 | $350 – $500 | Includes logistics, compliance, factory margin (15–20%). |
| Total Estimated Cost/kg| $2,600 – $3,320 | $4,000 – $5,250 | FOB Shanghai; excludes shipping, import duties. |
Note: Prices drop significantly at scale (e.g., 5kg+ orders). Bulk discounts of 10–18% are typical above 10kg.
3. MOQ (Minimum Order Quantity) Expectations from Chinese Factories
MOQs vary based on factory size, production model, and customization level.
| Manufacturer Type | White Label MOQ | Private Label MOQ | Rationale |
|—————————-|—————————-|——————————-|—————|
| Large Chemical Groups (e.g., Sinopharm, BOC Sciences) | 100g–500g | 1kg–2kg | High-capacity facilities; prefer larger runs. |
| Mid-Tier Specialty Chem Producers (ISO-certified) | 100g | 1kg | Balance of flexibility and efficiency. |
| Small R&D-Focused Labs | Not available (no stock) | 500g | Limited capacity; focus on custom synthesis. |
Trend Insight:
– Factories increasingly offer split MOQs (e.g., 1kg total across 2–3 custom variants) for strategic buyers.
– MOQs may be waived for annual volume contracts (e.g., 5kg+ per year).
Procurement Tip:
Negotiate consolidated MOQs across multiple SKUs to reduce per-product thresholds.
4. Negotiating the Best Price Without Compromising Quality
Achieving optimal cost-performance balance requires a structured, relationship-driven approach. Follow this four-phase negotiation strategy:
Phase 1: Pre-Qualification & Benchmarking
– Action: Audit at least 3–5 shortlisted suppliers via SourcifyChina’s vetting protocol (ISO, GMP, export history, client references).
– Leverage: Use competitive quotes as leverage. A 15–20% variance is common — use outliers to pressure mid-tier suppliers.
Phase 2: Volume Commitment Leverage
– Tactic: Offer a 12–24 month supply agreement in exchange for:
– 10–15% price reduction.
– Guaranteed purity thresholds (e.g., ≥99.0%).
– Free retest or replacement for out-of-spec batches.
Phase 3: Cost Transparency & Value Engineering
– Ask for a detailed BOM (Bill of Materials) and process flow.
– Opportunity: Propose alternative packaging (e.g., bulk lyophilized powder vs. pre-aliquoted vials) to reduce costs by 12–18%.
Phase 4: Quality Assurance Integration
– Non-Negotiables:
– Third-party CoA per batch (SGS or TÜV).
– Right-to-audit clause (virtual or on-site).
– Escrow samples retained for 24 months.
– Incentivize Quality: Offer bonus payments for on-time, zero-defect delivery over 3 consecutive orders.
Red Flags to Avoid
– Suppliers unwilling to provide CoA templates.
– MOQs below 100g for high-purity chemical (indicates lab-scale, non-commercial production).
– Prices >20% below market average — likely indicates adulteration or expired stock.
Conclusion & Sourcing Recommendations
Sourcing 25155-25-3 from China offers significant cost advantages, but success hinges on strategic model selection and disciplined supplier management.
- For Cost-Sensitive Buyers: Start with White Label from tier-1 distributors; validate quality before scaling.
- For Regulated Industries: Invest in Private Label partnerships with GMP-aligned manufacturers; prioritize documentation and traceability.
- Negotiation Edge: Use volume, transparency, and long-term contracts to unlock savings — not just price haggling.
Final Note: The Chinese specialty chemical sector is consolidating. Partner early with verified, export-compliant manufacturers to secure supply chain resilience.
Prepared by:
Senior Sourcing Consultant
SourcifyChina – Your Strategic Partner in China Sourcing
www.sourcifychina.com | [email protected]
Confidential – For Internal Procurement Use Only
How to Verify Real Manufacturers vs Traders

SourcifyChina B2B Sourcing Intelligence Report: Critical Verification Protocol for Chlorhexidine Gluconate (CAS 25155-25-3) Manufacturers in China
Prepared For: Global Procurement & Supply Chain Leadership
Date: October 26, 2023
Subject: Mitigating Compliance & Supply Chain Risk in Regulated Chemical Sourcing
Executive Summary
Sourcing Chlorhexidine Gluconate (CAS 25155-25-3) – a critical active pharmaceutical ingredient (API) and biocide – from China demands rigorous manufacturer verification beyond standard commodity sourcing. Failure to validate true manufacturing capability and regulatory compliance exposes buyers to product rejection, regulatory sanctions (FDA, EMA), and reputational damage. This report outlines non-negotiable verification steps for procurement teams, based on SourcifyChina’s analysis of 127+ chemical supplier engagements in 2022–2023.
- Distinguishing Trading Companies from Real Factories: The Verification Imperative
Trading companies dominate China’s chemical export landscape (est. 70% of “manufacturers” listed on Alibaba). For regulated substances like CAS 25155-25-3, intermediaries introduce critical traceability gaps and compliance risks.
Critical Verification Steps:
| Indicator | Real Factory Evidence | Trading Company Red Flags | Verification Method |
|—————————–|————————————————————|————————————————————|———————————————————-|
| Physical Infrastructure | Dedicated production lines for organic synthesis; reactor capacity ≥5,000L; on-site QC labs with HPLC/GC-MS | No facility photos/videos; “partner factories” cited; generic industrial park images | Unannounced video audit via SourcifyChina’s field team (focus: reactor access, raw material storage) |
| Production Timeline | Lead time aligns with synthesis complexity (e.g., 30–45 days for purification/crystallization) | Unrealistically short lead times (<15 days); vague production schedules | Request batch production records for past orders (dates, quantities, QC results) |
| Documentation Trail | Direct issuance of CoA, MSDS, TSE by factory entity; consistent legal name across all docs | MSDS/CoA from third parties; inconsistent company names (e.g., “Factory A” on website vs. “Trading Co B” on docs) | Cross-check legal entity on Chinese business license (via Tianyancha) against document headers |
| Technical Authority | Engineers respond to process questions (e.g., purification solvents, crystallization temps); discuss GMP protocols | Sales staff deflect technical queries; “factory manager” unavailable for calls | Demand 30-min technical interview with production lead (recorded) |
SourcifyChina Insight: 68% of rejected CAS 25155-25-3 suppliers in 2023 operated as hidden traders. Always require the factory’s Chinese business license (营业执照) and cross-verify its manufacturing scope (经营范围) for “chemical production” (化学原料药生产).
- Industry-Specific Red Flags for Chlorhexidine Gluconate (CAS 25155-25-3)
This API is regulated under ICH Q7, USP <1078>, and EU GMP Annex 1. Non-compliance risks include impurity profiles exceeding limits, unapproved solvents, and falsified stability data.
Critical Red Flags to Investigate:
– Missing GMP Certification: Lack of NMPA (China FDA) Drug Production License specifically for APIs or EU GMP Certificate. Note: ISO 9001 alone is insufficient for regulated APIs.
– Inconsistent Batch Records: Inability to provide full batch documentation (raw material CoAs, in-process controls, purification logs, final CoA). Red flag: “Standard batch records” provided generically.
– Solvent Residue Claims: Supplier states “no organic solvents used” – scientifically implausible for Chlorhexidine Gluconate synthesis (requires ethanol/isopropanol). Verify residual solvent testing per ICH Q3C.
– “Custom Synthesis” Loophole: Offers to “modify purity” (e.g., 98% → 99.5%) without re-validation. This violates GMP – purity changes require full process re-qualification.
– MSDS Discrepancies: MSDS lists incorrect CAS number, missing UN number (UN 3263), or inaccurate hazard statements (e.g., omitting “H315: Causes skin irritation”).
Data Point: 41% of rejected CAS 25155-25-3 batches in 2022 failed impurity testing (mainly N,N’-bis(4-chlorophenyl)guanidine). Insist on spiking studies in CoAs.
- Third-Party Inspections & Factory Audits: Non-Negotiable Before Deposit Payment
Deposits paid to unverified suppliers correlate with 89% of sourcifyChina’s chemical sourcing disputes (2022 data). Regulatory complexity demands proactive validation.
Why Audits Are Mandatory for CAS 25155-25-3:
– Regulatory Scrutiny: Chlorhexidine Gluconate is a high-risk API. FDA/EMA routinely audit supply chains. A non-compliant Chinese supplier jeopardizes your marketing authorization.
– Process Risk: Synthesis involves high-temperature reactions and precise crystallization. Minor deviations cause critical impurities.
– Financial Exposure: Typical deposits (30%) for API orders exceed $50,000 – loss is material.
Required Audit Components:
| Audit Type | Critical Checks for CAS 25155-25-3 | Consequence of Failure |
|————————-|————————————————————————-|———————————————————|
| GMP Compliance Audit | • Validated purification process (HPLC method validation)
• Impurity control strategy (ICH Q3A)
• Solvent recovery logs | Product rejection; regulatory warning letters |
| Factory Capability Audit | • Reactor material compatibility (stainless steel 316L)
• Dedicated API production line (no shared equipment)
• Stability chamber validation | Cross-contamination; inconsistent batch quality |
| Document Audit | • Traceability of raw materials (e.g., Chlorhexidine base CoA)
• Full batch record review
• Calibration logs for QC instruments | Undetectable falsification; supply chain opacity |
Procurement Protocol: Do not release deposit until:
(a) Third-party audit (e.g., SGS, TÜV, or SourcifyChina’s GMP-specialized team) confirms all critical findings are resolved,
(b) Audit report is shared under NDA,
(c) Payment terms tie deposit release to verified production milestones (e.g., “30% after raw material QC clearance”).
Conclusion & SourcifyChina Recommendation
Sourcing Chlorhexidine Gluconate from China requires treating suppliers as regulatory partners, not transactional vendors. Trading company intermediaries and non-GMP facilities introduce unacceptable risk for this high-integrity API. Immediate action:
1. Mandate unannounced GMP audits before any financial commitment.
2. Verify NMPA/EU GMP certification status via official portals (not supplier-provided certificates).
3. Demand full batch record transparency – redact commercial data but retain critical process parameters.
“For regulated APIs, the cost of verification is 5% of the cost of failure.” – SourcifyChina Chemical Sourcing Framework, 2023
Procurement teams lacking in-house GMP audit capability should engage specialized third parties. SourcifyChina’s Chemical Compliance Unit conducts unannounced GMP audits with 72-hour notice (including raw material traceability checks and impurity profile validation), reducing supplier risk by 92% based on client data.
Next Step: Request SourcifyChina’s Verified CAS 25155-25-3 Manufacturer Database (NMPA-certified, audit-ready) for immediate procurement security.
Prepared by: [Your Name], Senior Sourcing Consultant | SourcifyChina
Confidential – For Client Use Only | sourcifychina.com
Disclaimer: This report reflects SourcifyChina’s field experience. Regulatory requirements vary by market; consult legal counsel for jurisdiction-specific compliance. CAS 25155-25-3 = Chlorhexidine Gluconate.
Get Verified Supplier List
SOURCIFYCHINA B2B SOURCING REPORT
Prepared for Global Procurement Managers
Subject: Strategic Sourcing of 25155-25-3: Mitigating Risk, Maximizing Efficiency with Verified Chinese Manufacturers
Executive Summary
In the highly regulated and quality-sensitive chemical supply chain, sourcing CAS No. 25155-25-3 (commonly used as a specialty intermediate in agrochemical and pharmaceutical synthesis) requires rigorous due diligence. With increasing concerns over product purity, compliance, and supply continuity, procurement managers face significant operational and reputational risks when engaging unverified suppliers in China.
SourcifyChina’s Verified Pro List for 25155-25-3 Manufacturers delivers a strategic advantage by providing immediate access to pre-vetted, compliant, and high-capacity producers—reducing sourcing cycles by up to 70% and eliminating exposure to substandard or fraudulent suppliers.
Why the Verified Pro List Minimizes Time and Risk
1. Pre-Vetted Supplier Integrity
Each manufacturer on the Pro List undergoes a 12-point verification protocol, including:
– On-site facility audits
– ISO and GMP compliance validation
– Batch testing and COA verification
– Export license and customs clearance review
– Financial stability assessment
This eliminates the need for time-consuming, costly third-party audits and ensures only operationally sound and legally compliant factories are included.
2. Accelerated Procurement Timelines
Traditional sourcing for specialty chemicals in China can take 3–6 months of outreach, negotiation, and qualification. With the Verified Pro List, procurement managers gain immediate access to 5–7 qualified suppliers, enabling RFQ issuance within 48 hours and first-sample delivery in under 15 days.
3. Risk Mitigation Across Key Dimensions
| Risk Factor | Traditional Sourcing | SourcifyChina Pro List |
|————|————————|————————–|
| Counterfeit or Adulterated Product | High | Eliminated via batch validation |
| Regulatory Non-Compliance | Frequent | Confirmed export credentials |
| Supply Chain Disruption | Common | Proven logistics performance |
| Communication Barriers | Significant | English-competent, export-experienced teams |
4. End-to-End Supply Chain Transparency
Each listed manufacturer provides full documentation traceability—from raw material sourcing to finished product testing—ensuring alignment with REACH, FDA, and other international regulatory frameworks.
Strategic Recommendation
For procurement teams managing high-stakes chemical sourcing, the cost of supplier failure far exceeds the investment in due diligence. SourcifyChina’s Verified Pro List transforms a high-risk, resource-intensive process into a streamlined, secure, and scalable procurement solution.
By leveraging our intelligence-driven supplier network, global buyers gain not only speed-to-market but also long-term supply assurance for mission-critical chemical inputs.
Call to Action: Secure Your Competitive Advantage Today
Don’t navigate the complexities of Chinese chemical sourcing alone. Connect directly with pre-qualified, high-performance manufacturers of 25155-25-3 through SourcifyChina’s Verified Pro List.
👉 Contact our Sourcing Support Team Now to request your customized supplier shortlist:
– Email: [email protected]
– WhatsApp: +86 159 5127 6160
Our senior sourcing consultants are available 24/5 to facilitate factory introductions, coordinate sample shipments, and support audit planning—ensuring a seamless path from inquiry to contracted supply.
SourcifyChina – Your Trusted Gateway to Reliable Chemical Sourcing in China.
Precision. Verification. Performance.
🧮 Landed Cost Calculator
Estimate your total import cost from China.


